PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer
- PMID: 31028147
- PMCID: PMC6525547
- DOI: 10.1073/pnas.1822001116
PD-1+ regulatory T cells amplified by PD-1 blockade promote hyperprogression of cancer
Abstract
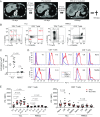
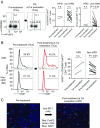
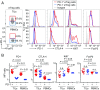
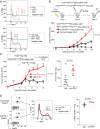
PD-1 blockade is a cancer immunotherapy effective in various types of cancer. In a fraction of treated patients, however, it causes rapid cancer progression called hyperprogressive disease (HPD). With our observation of HPD in ∼10% of anti-PD-1 monoclonal antibody (mAb)-treated advanced gastric cancer (GC) patients, we explored how anti-PD-1 mAb caused HPD in these patients and how HPD could be treated and prevented. In the majority of GC patients, tumor-infiltrating FoxP3highCD45RA-CD4+ T cells [effector Treg (eTreg) cells], which were abundant and highly suppressive in tumors, expressed PD-1 at equivalent levels as tumor-infiltrating CD4+ or CD8+ effector/memory T cells and at much higher levels than circulating eTreg cells. Comparison of GC tissue samples before and after anti-PD-1 mAb therapy revealed that the treatment markedly increased tumor-infiltrating proliferative (Ki67+) eTreg cells in HPD patients, contrasting with their reduction in non-HPD patients. Functionally, circulating and tumor-infiltrating PD-1+ eTreg cells were highly activated, showing higher expression of CTLA-4 than PD-1- eTreg cells. PD-1 blockade significantly enhanced in vitro Treg cell suppressive activity. Similarly, in mice, genetic ablation or antibody-mediated blockade of PD-1 in Treg cells increased their proliferation and suppression of antitumor immune responses. Taken together, PD-1 blockade may facilitate the proliferation of highly suppressive PD-1+ eTreg cells in HPDs, resulting in inhibition of antitumor immunity. The presence of actively proliferating PD-1+ eTreg cells in tumors is therefore a reliable marker for HPD. Depletion of eTreg cells in tumor tissues would be effective in treating and preventing HPD in PD-1 blockade cancer immunotherapy.
Keywords: PD-1; hyperprogressive disease; immune-checkpoint blockade; regulatory T cells.
Copyright © 2019 the Author(s). Published by PNAS.
Conflict of interest statement
Conflict of interest statement: The Sponsor declares a conflict of interest. Y. Togashi has received honoraria and grants from Ono Pharmaceutical as to this work, honoraria and grants from Bristol-Myers Squibb and AstraZeneca, and honoraria from Chugai Pharmaceutical and Merck Sharp & Dohme (MSD) outside of this study. K.S. received honoraria and grants from Ono Pharmaceutical and Bristol-Myers Squibb and grants from MSD outside of this study. H.N. received honoraria and grants from Ono Pharmaceutical as to this work, honoraria and grants from Bristol-Myers Squibb and Chugai Pharmaceutical, and grants from Taiho Pharmaceutical, Daiichi-Sankyo, Kyowa-Hakko Kirin, Zenyaku Kogyo, Astellas Pharmaceutical, Sysmex, and BD Japan outside of this study. Other authors declare no competing financial interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials
Miscellaneous

