Mass spectrometry-based molecular mapping of native FXIIIa cross-links in insoluble fibrin clots
- PMID: 31028172
- PMCID: PMC6552431
- DOI: 10.1074/jbc.AC119.007981
Mass spectrometry-based molecular mapping of native FXIIIa cross-links in insoluble fibrin clots
Abstract
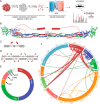
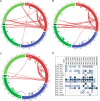
The roles of factor XIIIa-specific cross-links in thrombus formation, regression, or probability for embolization are largely unknown. A molecular understanding of fibrin architecture at the level of these cross-links could inform the development of therapeutic strategies to prevent the sequelae of thromboembolism. Here, we present an MS-based method to map native factor XIIIa cross-links in the insoluble matrix component of whole-blood or plasma-fibrin clots and in in vivo thrombi. Using a chaotrope-insoluble digestion method and quantitative cross-linking MS, we identified the previously mapped fibrinogen peptides that are responsible for covalent D-dimer association, as well as dozens of novel cross-links in the αC region of fibrinogen α. Our findings expand the known native cross-linked species from one to over 100 and suggest distinct antiparallel registries for interprotofibril association and covalent attachment of serpins that regulate clot dissolution.
Keywords: chemical digestion; factor XIII; fibrin; fibrinolysis; mass spectrometry (MS); protein cross-linking; thrombosis; transglutaminase.
© 2019 Schmitt et al.
Conflict of interest statement
The authors declare that they have no conflicts of interest with the contents of this article
Figures


References
-
- Collet J.-P., Moen J. L., Veklich Y. I., Gorkun O. V., Lord S. T., Montalescot G., and Weisel J. W. (2005) The αC domains of fibrinogen affect the structure of the fibrin clot, its physical properties, and its susceptibility to fibrinolysis. Blood 106, 3824–3830 10.1182/blood-2005-05-2150 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources

