Protective Role of Peroxiredoxin I in Heat-Killed Staphylococcus Aureus-infected Mice
- PMID: 31028193
- PMCID: PMC6559896
- DOI: 10.21873/invivo.11535
Protective Role of Peroxiredoxin I in Heat-Killed Staphylococcus Aureus-infected Mice
Abstract
Background/aim: Staphylococcus aureus (S. aureus) is a major gram-positive pathogen, which can cause toxic and immunogenic injuries both in nosocomial and community-acquired infections. Peroxiredoxin (Prx) I plays crucial roles in cellular apoptosis, proliferation, and signal transduction as well as in immunoregulation. The present study aimed to investigate whether Prx I protects mice from death caused by the heat-killed Staphylococcus aureus.
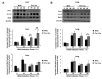
Materials and methods: In the present study, we challenged the wild-type and Prx I-deficient mice with heat-killed S. aureus (HKSA). The effects of Prx I were evaluated by a series of in vitro and in vivo experiments including western blot, Haematoxylin and Eosin staining, splenocyte analysis and cytokines analysis.
Results: Intra-peritoneal (ip) inoculation of HKSA resulted in increased mortality of Prx I-knockout (KO) mice with severe liver damage and highly populated spleens with lymphocytes. Furthermore, HKSA infections also bursted the production of both pro-inflammatory and anti-inflammatory serum cytokines in Prx I KO compared to wild-type mice.
Conclusion: Enhanced mortality of S. aureus-infected mice with Prx I deficiency suggested that Prx I may protect against the infection-associated lethality of mice.
Keywords: Peroxiredoxin I; Staphylococcus aureus; inflammatory; intra-peritoneal; knockout.
Copyright© 2019, International Institute of Anticancer Research (Dr. George J. Delinasios), All rights reserved.
Conflict of interest statement
The Authors declare that there are no conflicts of interest.
Figures



References
-
- Morinaka A, Funato Y, Uesugi K, Miki H. Oligomeric peroxiredoxin-i is an essential intermediate for p53 to activate mst1 kinase and apoptosis. Oncogene. 2011;30(40):4208–4218. PMID: 21516123. DOI: 10.1038/onc.2011.139. - PubMed
-
- Jarvis RM, Hughes SM, Ledgerwood EC. Peroxiredoxin 1 functions as a signal peroxidase to receive, transduce, and transmit peroxide signals in mammalian cells. Free Radic Biol Med. 2012;53(7):1522–1530. PMID: 22902630. DOI: 10.1016/ j.freeradbiomed.2012.08.001. - PubMed
-
- Lee SF, Pervaiz S. Assessment of oxidative stress-induced DNA damage by immunoflourescent analysis of 8-oxodg. Methods Cell Biol. 2011;103:99–113. PMID: 21722801 DOI: 10.1016/B978-0-12-385493-3.00005-X. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Research Materials
