UBXN1 interacts with the S1 protein of transmissible gastroenteritis coronavirus and plays a role in viral replication
- PMID: 31029162
- PMCID: PMC6487014
- DOI: 10.1186/s13567-019-0648-9
UBXN1 interacts with the S1 protein of transmissible gastroenteritis coronavirus and plays a role in viral replication
Abstract
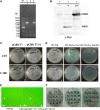
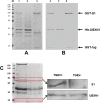
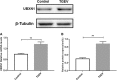
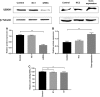
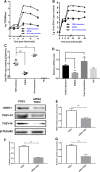
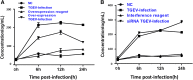
Transmissible gastroenteritis coronavirus (TGEV) is an enteropathogenic coronavirus that causes diarrhea in pigs and is associated with high morbidity and mortality in sucking piglets. S1 is one of two protein domains in the spike (S) glycoprotein and is responsible for enteric tropism, sialic acid recognition, and host receptor binding. Although there has been extensive research on the S1 protein of TGEV, little is known about the intracellular role of TGEV-S1. In the present study, we used yeast two-hybrid screening of a cDNA library from porcine intestinal cells to identify proteins that interact with TGEV-S1. Among 120 positive clones from the library, 12 intracellular proteins were identified after sequencing and a BLAST search. These intracellular proteins are involved in protein synthesis and degradation, biological signal transduction, and negative control of signaling pathways. Using a glutathione-S-transferase (GST) pulldown assay and Co-IP, we found that UBXN1 interacts with the S1 protein. Here, we observed that TGEV infection led to increased UBXN1 expression levels during the late phase of infection in IPEC-J2 cells. Inhibition of UBXN1 in IPEC-J2 cells via siRNA interference significantly decreased the viral titer and downregulated the expression of S1. UBXN1 overexpression significantly increased the viral copy number. Additionally, we provided data suggesting that UBXN1 negatively regulates IFN-β expression after TGEV infection. Finally, our research indicated that UBXN1 plays a vital role in the process of TGEV infection, making it a candidate target for the development of a novel antiviral method.
Figures
References
-
- Ahn DJ, Youm JW, Kim SW, Yoon WK, Kim HC, Hur TY, Joung YH, Jeon JH, Kim HS. Expression of the S glycoprotein of transmissible gastroenteritis virus (TGEV) in transgenic potato and its immunogenicity in mice. Korean J Vet Res. 2013;53:217–224. doi: 10.14405/kjvr.2013.53.4.217. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

