Silk fibroin scaffolds seeded with Wharton's jelly mesenchymal stem cells enhance re-epithelialization and reduce formation of scar tissue after cutaneous wound healing
- PMID: 31029166
- PMCID: PMC6487033
- DOI: 10.1186/s13287-019-1229-6
Silk fibroin scaffolds seeded with Wharton's jelly mesenchymal stem cells enhance re-epithelialization and reduce formation of scar tissue after cutaneous wound healing
Abstract
Background: The treatment of extensive and/or chronic skin wounds is a widespread and costly public health problem. Mesenchymal stem cells (MSCs) have been proposed as a potential cell therapy for inducing wound healing in different clinical settings, alone or in combination with biosynthetic scaffolds. Among them, silk fibroin (SF) seeded with MSCs has been shown to have increased efficacy in skin wound healing experimental models.
Methods: In this report, we investigated the wound healing effects of electrospun SF scaffolds cellularized with human Wharton's jelly MSCs (Wj-MSCs-SF) using a murine excisional wound splinting model.
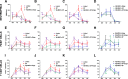
Results: Immunohistopathological examination after transplant confirmed the presence of infiltrated human fibroblast-like CD90-positive cells in the dermis of the Wj-MSCs-SF-treated group, yielding neoangiogenesis, decreased inflammatory infiltrate and myofibroblast proliferation, less collagen matrix production, and complete epidermal regeneration.
Conclusions: These findings indicate that Wj-MSCs transplanted in the wound bed on a silk fibroin scaffold contribute to the generation of a well-organized and vascularized granulation tissue, enhance reepithelization of the wound, and reduce the formation of fibrotic scar tissue, highlighting the potential therapeutic effects of Wj-MSC-based tissue engineering approaches to non-healing wound treatment.
Keywords: Mesenchymal stem cells; Silk fibroin; Wharton’s jelly; Wound healing.
Conflict of interest statement
Ethics approval and consent to participate
This study was approved by the ethics committee of Hospital Clinico Universitario Virgen de la Arrixaca (Murcia, Spain). Umbilical cord donors provided written and informed consent in accordance with the Declaration of Helsinki. All procedures involving animals were previously approved by the University of Murcia animal care committee and conducted in accordance with the national guidelines on animal care.
Consent for publication
Not applicable
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures






References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

