Targeted capture-based NGS is superior to multiplex PCR-based NGS for hereditary BRCA1 and BRCA2 gene analysis in FFPE tumor samples
- PMID: 31029168
- PMCID: PMC6487025
- DOI: 10.1186/s12885-019-5584-6
Targeted capture-based NGS is superior to multiplex PCR-based NGS for hereditary BRCA1 and BRCA2 gene analysis in FFPE tumor samples
Abstract
Background: With the introduction of Olaparib treatment for BRCA-deficient recurrent ovarian cancer, testing for somatic and/or germline mutations in BRCA1/2 genes in tumor tissues became essential for treatment decisions. In most cases only formalin-fixed paraffin-embedded (FFPE) samples, containing fragmented and chemically modified DNA of minor quality, are available. Thus, multiplex PCR-based sequencing is most commonly applied in routine molecular testing, which is predominantly focused on the identification of known hot spot mutations in oncogenes.
Methods: We compared the overall performance of an adjusted targeted capture-based enrichment protocol and a multiplex PCR-based approach for calling of pathogenic SNVs and InDels using DNA extracted from 13 FFPE tissue samples. We further applied both strategies to seven blood samples and five matched FFPE tumor tissues of patients with known germline exon-spanning deletions and gene-wide duplications in BRCA1/2 to evaluate CNV detection based solely on panel NGS data. Finally, we analyzed DNA from FFPE tissues of 11 index patients from families suspected of having hereditary breast and ovarian cancer, of whom no blood samples were available for testing, in order to identify underlying pathogenic germline BRCA1/2 mutations.
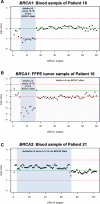
Results: The multiplex PCR-based protocol produced inhomogeneous coverage among targets of each sample and between samples as well as sporadic amplicon drop out, leading to insufficiently or non-covered nucleotides, which subsequently hindered variant detection. This protocol further led to detection of PCR-artifacts that could easily have been misinterpreted as pathogenic mutations. No such limitations were observed by application of an adjusted targeted capture-based protocol, which allowed for CNV calling with 86% sensitivity and 100% specificity. All pathogenic CNVs were confirmed in the five matched FFPE tumor samples from patients carrying known pathogenic germline mutations and we additionally identified somatic loss of the second allele in BRCA1/2. Furthermore we detected pathogenic BRCA1/2 variants in four the eleven FFPE samples from patients of whom no blood was available for analysis.
Conclusions: We demonstrate that an adjusted targeted capture-based enrichment protocol is superior to commonly applied multiplex PCR-based protocols for reliable BRCA1/2 variant detection, including CNV-detection, using FFPE tumor samples.
Keywords: BRCA1; BRCA2; CNV detection; FFPE tissue; Genetic testing; HBOC; NGS; Pathogenic germline mutations; Targeted capture-based NGS.
Conflict of interest statement
Ethics approval and consent to participate
This research has been approved by the Ethics Committee of Medizinische Fakultät der Technischen Universität Dresden by “Ergebnisforschung im Kontext des Familiären Brust- und Eierstockkrebs” der Klinik und Poliklinik für Frauenheilkunde und Geburtshilfe des Universitätsklinikums Dresden/Medizinische Fakultät “Carl Gustav Carus”, Technischen Universität Dresden" (reference number: EK162072007) and by "Feingewebliche, immunohistologische und molekularpathologische Untersuchungen in langzeit-archiviertem Gewebsmaterial des Institutes für Pathologie des Universitätsklinikums Dresden/Medizinische Fakultät “Carl Gustav Carus”, Technischen Universität Dresden "(reference number: EK59032007). The Ethics Committee of Medizinische Fakultät der Technischen Universität Dresden bases its assessment of the submitted study on the guidelines of the revised declaration of the World Medical Association of Helsinki in the respectively valid version, on the Pharmaceutical Code, on the Radiation Protection Ordinance and on the generally recognized guidelines for “ICH-GCP E6”. The authors confirm that a written declaration of consent has been obtained from all patients prior to the genetic examination according to the German Gene Diagnosis Act (GenDG). No identifiable personal patient data is displayed in this article.
Consent for publication
Written consent to publish family trees displayed in Fig. 4 of this work has been obtained from respective participants.
Competing interests
PW is advisory board member of AstraZeneca and got honoraria for scientific talks.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures



Similar articles
-
BRCA somatic and germline mutation detection in paraffin embedded ovarian cancers by next-generation sequencing.Oncotarget. 2016 Jan 12;7(2):1076-83. doi: 10.18632/oncotarget.6834. Oncotarget. 2016. PMID: 26745875 Free PMC article.
-
Simultaneous detection of BRCA mutations and large genomic rearrangements in germline DNA and FFPE tumor samples.Oncotarget. 2016 Sep 20;7(38):61845-61859. doi: 10.18632/oncotarget.11259. Oncotarget. 2016. PMID: 27533253 Free PMC article.
-
BRCA1/2 somatic mutation detection in formalin-fixed paraffin embedded tissue by next-generation sequencing in Korean ovarian cancer patients.Pathol Res Pract. 2019 Nov;215(11):152595. doi: 10.1016/j.prp.2019.152595. Epub 2019 Aug 16. Pathol Res Pract. 2019. PMID: 31570282
-
[Testing of mutations in BRCA1 and BRCA2 genes in tumor tissues - possibilities and limitations].Cesk Patol. 2016 Fall;52(4):210-214. Cesk Patol. 2016. PMID: 27869447 Review. Czech.
-
[Detecting Large Germline Rearrangements of BRCA1 by Next Generation Tumor Sequencing].Mol Biol (Mosk). 2020 May-Jun;54(4):688-698. doi: 10.31857/S0026898420040114. Mol Biol (Mosk). 2020. PMID: 32840490 Review. Russian.
Cited by
-
Novel CRISPR-based sequence specific enrichment methods for target loci and single base mutations.PLoS One. 2020 Dec 23;15(12):e0243781. doi: 10.1371/journal.pone.0243781. eCollection 2020. PLoS One. 2020. PMID: 33362267 Free PMC article.
-
Target Enrichment Approaches for Next-Generation Sequencing Applications in Oncology.Diagnostics (Basel). 2022 Jun 24;12(7):1539. doi: 10.3390/diagnostics12071539. Diagnostics (Basel). 2022. PMID: 35885445 Free PMC article. Review.
-
Ultrasensitive detection of tumor-specific mutations in saliva of patients with oral cavity squamous cell carcinoma.Cancer. 2021 May 15;127(10):1576-1589. doi: 10.1002/cncr.33393. Epub 2020 Dec 21. Cancer. 2021. PMID: 33405231 Free PMC article.
-
BRCA1/2 Testing Landscape in Ovarian Cancer: A Nationwide, Real-World Data Study.Cancers (Basel). 2024 Apr 26;16(9):1682. doi: 10.3390/cancers16091682. Cancers (Basel). 2024. PMID: 38730634 Free PMC article.
-
Comparison of Homologous Recombination Repair Gene Next-Generation Sequencing Analysis in Patients With Metastatic Castration-Resistant Prostate Cancer Between Local and Central Laboratories in Korea.Ann Lab Med. 2023 Jan 1;43(1):64-72. doi: 10.3343/alm.2023.43.1.64. Epub 2022 Sep 1. Ann Lab Med. 2023. PMID: 36045058 Free PMC article.
References
-
- Schroeder C, Faust U, Sturm M, Hackmann K, Grundmann K, Harmuth F, et al. HBOC multi-gene panel testing: comparison of two sequencing centers. Breast Cancer Res. Treat. [Internet]. 2015 [cited 2017 Apr 12];152:129–36. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26022348 - PubMed
-
- Kuchenbaecker KB, Hopper JL, Barnes DR, Phillips K-A, Mooij TM, Roos-Blom M-J, et al. Risks of Breast, Ovarian, and Contralateral Breast Cancer for BRCA1 and BRCA2 Mutation Carriers. JAMA [Internet]. American Medical Association; 2017 [cited 2018 Jan 29];317:2402. Available from: http://jama.jamanetwork.com/article.aspx?doi=10.1001/jama.2017.7112 - PubMed
-
- Kast K, Rhiem K, Wappenschmidt B, Hahnen E, Hauke J, Bluemcke B, et al. Prevalence of BRCA1/2 germline mutations in 21 401 families with breast and ovarian cancer. J. Med. Genet. [Internet]. 2016 [cited 2018 Jan 29];53:465–71. Available from: http://www.ncbi.nlm.nih.gov/pubmed/26928436 - PubMed
-
- Wang W. Emergence of a DNA-damage response network consisting of Fanconi anaemia and BRCA proteins. Nat. Rev. Genet. [Internet]. 2007 [cited 2017 Apr 18];8:735–48. Available from: http://www.ncbi.nlm.nih.gov/pubmed/17768402 - PubMed
-
- Gudmundsdottir K, Ashworth A. The roles of BRCA1 and BRCA2 and associated proteins in the maintenance of genomic stability. Oncogene [Internet]. 2006 [cited 2017 Apr 18];25:5864–74. Available from: http://www.ncbi.nlm.nih.gov/pubmed/16998501 - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

