Novel parietal epithelial cell subpopulations contribute to focal segmental glomerulosclerosis and glomerular tip lesions
- PMID: 31029503
- PMCID: PMC7292612
- DOI: 10.1016/j.kint.2019.01.037
Novel parietal epithelial cell subpopulations contribute to focal segmental glomerulosclerosis and glomerular tip lesions
Abstract
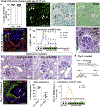
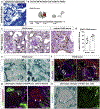
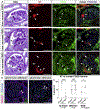
Beside the classical flat parietal epithelial cells (PECs), we investigated proximal tubular epithelial-like cells, a neglected subgroup of PECs. These cells, termed cuboidal PECs, make up the most proximal part of the proximal tubule and may also line parts of Bowman's capsule. Additionally, a third intermediate PEC subgroup was identified at the junction between the flat and cuboidal PEC subgroups at the tubular orifice. The transgenic mouse line PEC-rtTA labeled all three PEC subgroups. Here we show that the inducible Pax8-rtTA mouse line specifically labeled only cuboidal and intermediate PECs, but not flat PECs. In aging Pax8-rtTA mice, cell fate mapping showed no evidence for significant transdifferentiation from flat PECs to cuboidal or intermediate PECs or vice versa. In murine glomerular disease models of crescentic glomerulonephritis, and focal segmental glomerulosclerosis (FSGS), intermediate PECs became more numerous. These intermediate PECs preferentially expressed activation markers CD44 and Ki-67, suggesting that this subgroup of PECs was activated more easily than the classical flat PECs. In mice with FSGS, cuboidal and intermediate PECs formed sclerotic lesions. In patients with FSGS, cells forming the tip lesions expressed markers of intermediate PECs. These novel PEC subgroups form sclerotic lesions and were more prone to cellular activation compared to the classical flat PECs in disease. Thus, colonization of Bowman's capsule by cuboidal PECs may predispose to lesion formation and chronic kidney disease. We propose that tip lesions originate from this novel subgroup of PECs in patients with FSGS.
Keywords: Columbia classification; FSGS; glomerular disease; parietal epithelial cells; tip lesions.
Copyright © 2019 International Society of Nephrology. Published by Elsevier Inc. All rights reserved.
Conflict of interest statement
DISCLOSURE
All the authors declared no competing interests.
Figures




Comment in
-
Recognizing diversity in parietal epithelial cells.Kidney Int. 2019 Jul;96(1):16-19. doi: 10.1016/j.kint.2019.02.036. Kidney Int. 2019. PMID: 31229027
-
Properties of intermediate parietal epithelial cells of Bowman's capsule.Kidney Int. 2019 Nov;96(5):1240-1241. doi: 10.1016/j.kint.2019.08.015. Kidney Int. 2019. PMID: 31648700 No abstract available.
-
The authors reply.Kidney Int. 2019 Nov;96(5):1241. doi: 10.1016/j.kint.2019.08.016. Kidney Int. 2019. PMID: 31648701 No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

