A biradical-tagged phospholipid as a polarizing agent for solid-state MAS Dynamic Nuclear Polarization NMR of membrane proteins
- PMID: 31029957
- PMCID: PMC6709687
- DOI: 10.1016/j.ssnmr.2019.04.003
A biradical-tagged phospholipid as a polarizing agent for solid-state MAS Dynamic Nuclear Polarization NMR of membrane proteins
Abstract
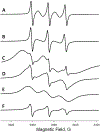
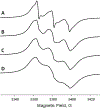
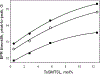
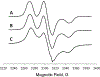
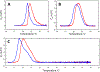
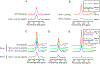
A novel Dynamic Nuclear Polarization (DNP) NMR polarizing agent ToSMTSL-PTE representing a phospholipid with a biradical TOTAPOL tethered to the polar head group has been synthesized, characterized, and employed to enhance solid-state Nuclear Magnetic Resonance (SSNMR) signal of a lipid-reconstituted integral membrane protein proteorhodopsin (PR). A matrix-free PR formulation for DNP improved the absolute sensitivity of NMR signal by a factor of ca. 4 compared to a conventional preparation with TOTAPOL dispersed in a glassy glycerol/water matrix. DNP enhancements measured at 400 MHz/263 GHz and 600 MHz/395 GHz showed a strong field dependence but remained moderate at both fields, and comparable to those obtained for PR covalently modified with ToSMTSL. Additional continuous wave (CW) X-band electron paramagnetic resonance (EPR) experiments with ToSMTSL-PTE in solutions and in lipid bilayers revealed that an unfavorable conformational change of the linker connecting mononitroxides could be one of the reasons for moderate DNP enhancements. Further, differential scanning calorimetry (DSC) and CW EPR experiments indicated an inhomogeneous distribution and/or a possibility of a partial aggregation of ToSMTSL-PTE in DMPC:DMPA bilayers when the concentration of the polarizing agent was increased to 20 mol% to maximize the DNP enhancement. Thus, conformational changes and an inhomogeneous distribution of the lipid-based biradicals in lipid bilayers emerged as important factors to consider for further development of this matrix-free approach for DNP of membrane proteins.
Keywords: Bilayer; Biradical; Dynamic nuclear polarization; Electron spin resonance; Lipid; Membrane protein; Nuclear magnetic resonance; Signal enhancement.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
Authors declare no competing financial interest.
Figures
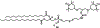
References
-
- Lilly Thankamony AS, Wittmann JJ, Kaushik M, Corzilius B, Dynamic nuclear polarization for sensitivity enhancement in modern solid-state NMR, Prog Nucl Magn Reson Spectrosc 102–103 (2017) 120–195. - PubMed
-
- Song CS, Hu KN, Joo CG, Swager TM, Griffin RG, TOTAPOL: A biradical polarizing agent for dynamic nuclear polarization experiments in aqueous media, J. Am. Chem. Soc 128(35) (2006) 11385–11390. - PubMed
-
- Jagtap AP, Geiger MA, Stoppler D, Orwick-Rydmark M, Oschkinat H, Sigurdsson ST, bcTol : a highly water-soluble biradical for efficient dynamic nuclear polarization of biomolecules, Chem Commun (Camb) 52(43) (2016) 7020–3. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

