A blood-based prognostic biomarker in IBD
- PMID: 31030191
- PMCID: PMC6691955
- DOI: 10.1136/gutjnl-2019-318343
A blood-based prognostic biomarker in IBD
Erratum in
-
Correction: A blood-based prognostic biomarker in IBD.Gut. 2019 Oct;68(10):1909-1912. doi: 10.1136/gutjnl-2019-318343corr1. Gut. 2019. PMID: 31492802 Free PMC article. No abstract available.
Abstract
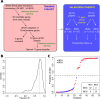
Objective: We have previously described a prognostic transcriptional signature in CD8 T cells that separates patients with IBD into two phenotypically distinct subgroups, termed IBD1 and IBD2. Here we sought to develop a blood-based test that could identify these subgroups without cell separation, and thus be suitable for clinical use in Crohn's disease (CD) and ulcerative colitis (UC).
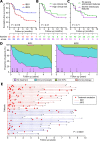
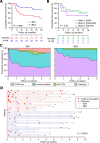
Design: Patients with active IBD were recruited before treatment. Transcriptomic analyses were performed on purified CD8 T cells and/or whole blood. Phenotype data were collected prospectively. IBD1/IBD2 patient subgroups were identified by consensus clustering of CD8 T cell transcriptomes. In a training cohort, machine learning was used to identify groups of genes ('classifiers') whose differential expression in whole blood recreated the IBD1/IBD2 subgroups. Genes from the best classifiers were quantitative (q)PCR optimised, and further machine learning was used to identify the optimal qPCR classifier, which was locked down for further testing. Independent validation was sought in separate cohorts of patients with CD (n=66) and UC (n=57).
Results: In both validation cohorts, a 17-gene qPCR-based classifier stratified patients into two distinct subgroups. Irrespective of the underlying diagnosis, IBDhi patients (analogous to the poor prognosis IBD1 subgroup) experienced significantly more aggressive disease than IBDlo patients (analogous to IBD2), with earlier need for treatment escalation (hazard ratio=2.65 (CD), 3.12 (UC)) and more escalations over time (for multiple escalations within 18 months: sensitivity=72.7% (CD), 100% (UC); negative predictive value=90.9% (CD), 100% (UC)).
Conclusion: This is the first validated prognostic biomarker that can predict prognosis in newly diagnosed patients with IBD and represents a step towards personalised therapy.
Keywords: Ibd basic besearch; Ibd clinical; crohn’s disease; gene expression; ulcerative colitis.
© Author(s) (or their employer(s)) 2019. Re-use permitted under CC BY. Published by BMJ.
Conflict of interest statement
Competing interests: DB, JCL, EM, PAL and KGCS are coinventors on a patent covering the method of assessing prognosis in IBD. EM, PAL and KGCS are cofounders and consultants for PredictImmune. JCL is a consultant for PredictImmune.
Figures

Comment in
-
Could a Blood Marker of T-Cell Exhaustion be Inflammatory Bowel Disease's New Crystal Ball?Gastroenterology. 2020 Mar;158(4):1168-1170. doi: 10.1053/j.gastro.2020.01.021. Epub 2020 Jan 15. Gastroenterology. 2020. PMID: 31954112 Free PMC article. No abstract available.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
