Role of Ryanodine Type 2 Receptors in Elementary Ca2+ Signaling in Arteries and Vascular Adaptive Responses
- PMID: 31030596
- PMCID: PMC6512102
- DOI: 10.1161/JAHA.118.010090
Role of Ryanodine Type 2 Receptors in Elementary Ca2+ Signaling in Arteries and Vascular Adaptive Responses
Abstract
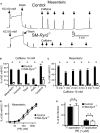
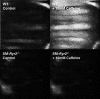
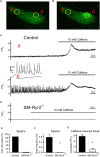
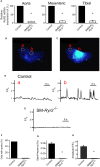
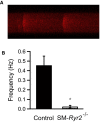
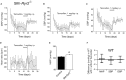
Background Hypertension is the major risk factor for cardiovascular disease, the most common cause of death worldwide. Resistance arteries are capable of adapting their diameter independently in response to pressure and flow-associated shear stress. Ryanodine receptors (RyRs) are major Ca2+-release channels in the sarcoplasmic reticulum membrane of myocytes that contribute to the regulation of contractility. Vascular smooth muscle cells exhibit 3 different RyR isoforms (RyR1, RyR2, and RyR3), but the impact of individual RyR isoforms on adaptive vascular responses is largely unknown. Herein, we generated tamoxifen-inducible smooth muscle cell-specific RyR2-deficient mice and tested the hypothesis that vascular smooth muscle cell RyR2s play a specific role in elementary Ca2+ signaling and adaptive vascular responses to vascular pressure and/or flow. Methods and Results Targeted deletion of the Ryr2 gene resulted in a complete loss of sarcoplasmic reticulum-mediated Ca2+-release events and associated Ca2+-activated, large-conductance K+ channel currents in peripheral arteries, leading to increased myogenic tone and systemic blood pressure. In the absence of RyR2, the pulmonary artery pressure response to sustained hypoxia was enhanced, but flow-dependent effects, including blood flow recovery in ischemic hind limbs, were unaffected. Conclusions Our results establish that RyR2-mediated Ca2+-release events in VSCM s specifically regulate myogenic tone (systemic circulation) and arterial adaptation in response to changes in pressure (hypoxic lung model), but not flow. They further suggest that vascular smooth muscle cell-expressed RyR2 deserves scrutiny as a therapeutic target for the treatment of vascular responses in hypertension and chronic vascular diseases.
Keywords: BKCa channel; Ca2+ sparks; blood pressure; hypoxia; isoforms; pulmonary hypertension; ryanodine receptors.
Figures






References
-
- Bevan JA, Garcia‐Roldan JL, Joyce EH. Resistance artery tone is influenced independently by pressure and by flow. Blood Vessels. 1990;27:202–207. - PubMed
-
- Nelson MT, Patlak JB, Worley JF, Standen NB. Calcium channels, potassium channels, and voltage dependence of arterial smooth muscle tone. Am J Physiol. 1990;259:C3–C18. - PubMed
-
- Wang SQ, Wei C, Zhao G, Brochet DX, Shen J, Song LS, Wang W, Yang D, Cheng H. Imaging microdomain Ca2+ in muscle cells. Circ Res. 2004;94:1011–1022. - PubMed
-
- Jaggar JH, Wellman GC, Heppner TJ, Porter VA, Perez GJ, Gollasch M, Kleppisch T, Rubart M, Stevenson AS, Lederer WJ, Knot HJ, Bonev AD, Nelson MT. Ca2+ channels, ryanodine receptors and Ca(2+)‐activated K+ channels: a functional unit for regulating arterial tone. Acta Physiol Scand. 1998;164:577–587. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Miscellaneous

