Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time
- PMID: 31030999
- PMCID: PMC6506277
- DOI: 10.1016/j.cell.2019.02.017
Insulin/IGF-1 Drives PERIOD Synthesis to Entrain Circadian Rhythms with Feeding Time
Abstract
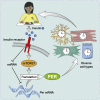
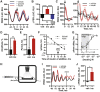
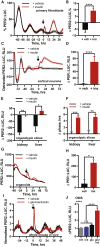
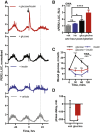
In mammals, endogenous circadian clocks sense and respond to daily feeding and lighting cues, adjusting internal ∼24 h rhythms to resonate with, and anticipate, external cycles of day and night. The mechanism underlying circadian entrainment to feeding time is critical for understanding why mistimed feeding, as occurs during shift work, disrupts circadian physiology, a state that is associated with increased incidence of chronic diseases such as type 2 (T2) diabetes. We show that feeding-regulated hormones insulin and insulin-like growth factor 1 (IGF-1) reset circadian clocks in vivo and in vitro by induction of PERIOD proteins, and mistimed insulin signaling disrupts circadian organization of mouse behavior and clock gene expression. Insulin and IGF-1 receptor signaling is sufficient to determine essential circadian parameters, principally via increased PERIOD protein synthesis. This requires coincident mechanistic target of rapamycin (mTOR) activation, increased phosphoinositide signaling, and microRNA downregulation. Besides its well-known homeostatic functions, we propose insulin and IGF-1 are primary signals of feeding time to cellular clocks throughout the body.
Keywords: IGF-1; PERIOD; circadian; food entrainment; insulin; mTORC1; miRNA.
Copyright © 2019 MRC Laboratory of Molecular Biology. Published by Elsevier Inc. All rights reserved.
Figures
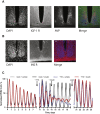

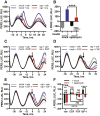


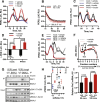

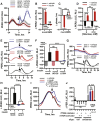



Comment in
-
Trained by feeding.Nat Rev Endocrinol. 2019 Jul;15(7):378. doi: 10.1038/s41574-019-0215-5. Nat Rev Endocrinol. 2019. PMID: 31086317 No abstract available.
References
-
- Abe H., Kida M., Tsuji K., Mano T. Feeding cycles entrain circadian rhythms of locomotor activity in CS mice but not in C57BL/6J mice. Physiol. Behav. 1989;45:397–401. - PubMed
-
- Anhê G.F., Caperuto L.C., Pereira-Da-Silva M., Souza L.C., Hirata A.E., Velloso L.A., Cipolla-Neto J., Carvalho C.R.O. In vivo activation of insulin receptor tyrosine kinase by melatonin in the rat hypothalamus. J. Neurochem. 2004;90:559–566. - PubMed
-
- Bae K., Weaver D.R. Transient, light-induced rhythmicity in mPer-deficient mice. J. Biol. Rhythms. 2007;22:85–88. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Miscellaneous

