Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish
- PMID: 31031007
- PMCID: PMC6570580
- DOI: 10.1016/j.cell.2019.04.004
Visualizing Engrafted Human Cancer and Therapy Responses in Immunodeficient Zebrafish
Abstract
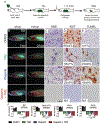
Xenograft cell transplantation into immunodeficient mice has become the gold standard for assessing pre-clinical efficacy of cancer drugs, yet direct visualization of single-cell phenotypes is difficult. Here, we report an optically-clear prkdc-/-, il2rga-/- zebrafish that lacks adaptive and natural killer immune cells, can engraft a wide array of human cancers at 37°C, and permits the dynamic visualization of single engrafted cells. For example, photoconversion cell-lineage tracing identified migratory and proliferative cell states in human rhabdomyosarcoma, a pediatric cancer of muscle. Additional experiments identified the preclinical efficacy of combination olaparib PARP inhibitor and temozolomide DNA-damaging agent as an effective therapy for rhabdomyosarcoma and visualized therapeutic responses using a four-color FUCCI cell-cycle fluorescent reporter. These experiments identified that combination treatment arrested rhabdomyosarcoma cells in the G2 cell cycle prior to induction of apoptosis. Finally, patient-derived xenografts could be engrafted into our model, opening new avenues for developing personalized therapeutic approaches in the future.
Keywords: SCID; breast cancer; il2rg; immune deficient; melanoma; prkdc; rhabdomyosarcoma; xenograft; zebrafish.
Copyright © 2019 Elsevier Inc. All rights reserved.
Figures



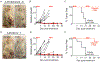

Comment in
-
Scientists See Human Cancer in Zebrafish.Cancer Discov. 2019 Jul;9(7):819-820. doi: 10.1158/2159-8290.CD-NB2019-062. Epub 2019 May 30. Cancer Discov. 2019. PMID: 31147317
-
Looking at xenografts in zebrafish.Nat Methods. 2019 Jul;16(7):578. doi: 10.1038/s41592-019-0485-9. Nat Methods. 2019. PMID: 31249414 No abstract available.
References
-
- Agarwala SS & Kirkwood JM (2000). Temozolomide, a novel alkylating agent with activity in the central nervous system, may improve the treatment of advanced metastatic melanoma. Oncologist, 5, 144–51. - PubMed
-
- Barr FG, Galili N, Holick J, Biegel JA, Rovera G & Emanuel BS (1993). Rearrangement of the PAX3 paired box gene in the paediatric solid tumour alveolar rhabdomyosarcoma. Nat Genet, 3, 113–7. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases
Research Materials

