Adaptive Immune Resistance Emerges from Tumor-Initiating Stem Cells
- PMID: 31031009
- PMCID: PMC6525024
- DOI: 10.1016/j.cell.2019.03.025
Adaptive Immune Resistance Emerges from Tumor-Initiating Stem Cells
Abstract
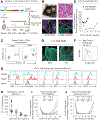
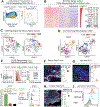
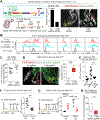
Our bodies are equipped with powerful immune surveillance to clear cancerous cells as they emerge. How tumor-initiating stem cells (tSCs) that form and propagate cancers equip themselves to overcome this barrier remains poorly understood. To tackle this problem, we designed a skin cancer model for squamous cell carcinoma (SCC) that can be effectively challenged by adoptive cytotoxic T cell transfer (ACT)-based immunotherapy. Using single-cell RNA sequencing (RNA-seq) and lineage tracing, we found that transforming growth factor β (TGF-β)-responding tSCs are superior at resisting ACT and form the root of tumor relapse. Probing mechanism, we discovered that during malignancy, tSCs selectively acquire CD80, a surface ligand previously identified on immune cells. Moreover, upon engaging cytotoxic T lymphocyte antigen-4 (CTLA4), CD80-expressing tSCs directly dampen cytotoxic T cell activity. Conversely, upon CTLA4- or TGF-β-blocking immunotherapies or Cd80 ablation, tSCs become vulnerable, diminishing tumor relapse after ACT treatment. Our findings place tSCs at the crux of how immune checkpoint pathways are activated.
Keywords: adoptive T cell transfer therapy; immune evasion; immune-stem cell interactions; lineage tracing; single-cell RNA sequencing; squamous cell carcinoma; tumor stem cell.
Copyright © 2019 Elsevier Inc. All rights reserved.
Conflict of interest statement
DECLARATION OF FINANCIAL INTERESTS.
The authors declare no competing financial interests.
Figures




References
-
- Alegre ML, Frauwirth KA, and Thompson CB (2001). T-cell regulation by CD28 and CTLA-4. Nat Rev Immunol 1, 220–228. - PubMed
-
- Batlle E, and Clevers H (2017). Cancer stem cells revisited. Nat Med 23, 1124–1134. - PubMed
-
- Beck B, Driessens G, Goossens S, Youssef KK, Kuchnio A, Caauwe A, Sotiropoulou PA, Loges S, Lapouge G, Candi A, et al. (2011). A vascular niche and a VEGF-Nrp1 loop regulate the initiation and stemness of skin tumours. Nature 478, 399–403. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases
Research Materials

