Postoperative Nausea and Vomiting Prophylaxis: A Comparative Study of Ramosetron and Palonosetron in Patients Undergoing Laparoscopic Cholecystectomy - A Prospective Randomized Trial
- PMID: 31031483
- PMCID: PMC6444952
- DOI: 10.4103/aer.AER_192_18
Postoperative Nausea and Vomiting Prophylaxis: A Comparative Study of Ramosetron and Palonosetron in Patients Undergoing Laparoscopic Cholecystectomy - A Prospective Randomized Trial
Abstract
Background: In spite of the availability of several antiemetic drugs, postoperative nausea and vomiting (PONV) is very common following laparoscopic surgery. Selective 5-hydroxytryptamine type 3 receptor antagonists are considered first-line agents for prophylaxis for PONV.
Aims: In this study, we investigated and compared the efficacy of ramosetron and palonosetron in preventing PONV following laparoscopic cholecystectomy.
Statistical analysis: The data were analyzed with Student's t-test and Chi-square test.
Settings and design: This was a randomized, prospective, double-blinded, observational clinical study.
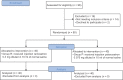
Methods: A total number of 80 patients, undergoing elective laparoscopic cholecystectomy surgeries under general anesthesia, were randomly assigned to one of the two equal groups to receive either of the following: Group R - received injection ramosetron 0.3 mg and Group P - received injection palonosetron 0.075 mg intravenous bolus immediately before the induction of anesthesia. The incidence of PONV, adverse effects of the study drugs, and need for rescue antiemetics were recorded over the next 48 h. Primary outcome was the incidence of PONV. Secondary outcomes were adverse effects of the study drugs and need for rescue.
Statistical analysis: The data were analyzed with Student's t-test and Chi-square test.
Results: The incidence of a complete response (no PONV and no rescue medication) during 0-3 h in the postoperative period was 82.5% with ramosetron and 90% with palonosetron; the incidence during 3-24 h postoperatively was 80% with ramosetron and 87.5% with palonosetron. During 24-48 h, the incidence was 65% and 90%, respectively (P < 0.05). The incidences of adverse effects were statistically insignificant between the groups.
Conclusion: Prophylactic therapy with palonosetron is more effective than ramosetron for long-term prevention of PONV following laparoscopic cholecystectomy.
Keywords: Antiemetics; ondansetron; palonosetron; postoperative nausea and vomiting.
Conflict of interest statement
There are no conflicts of interest.
Figures
References
-
- Kapur PA. The big “little problem”. Anesth Analg. 1991;73:243–5. - PubMed
-
- Gan TJ, Diemunsch P, Habib AS, Kovac A, Kranke P, Meyer TA, et al. Consensus guidelines for the management of postoperative nausea and vomiting. Anesth Analg. 2014;118:85–113. - PubMed
-
- Salmenperä M, Kuoppamäki R, Salmenperä A. Do anticholinergic agents affect the occurrence of postanaesthetic nausea? Acta Anaesthesiol Scand. 1992;36:445–8. - PubMed
-
- Islan S, Jain PN. PONV: A review article. Indian J Anaesth. 2004;48:253–8.
LinkOut - more resources
Full Text Sources
Other Literature Sources