A Straightforward Access to Stable, 16 Valence-electron Phosphine-Stabilized Fe0 Olefin Complexes and their Reactivity
- PMID: 31031510
- PMCID: PMC6485349
- DOI: 10.1021/acs.organomet.6b00803
A Straightforward Access to Stable, 16 Valence-electron Phosphine-Stabilized Fe0 Olefin Complexes and their Reactivity
Abstract
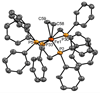
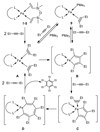
The use of the dialkene divinyltetramethyldisiloxane (dvtms) allows easy access to the reactive 16 valence-electron complexes [Fe0(L-L)(dvtms)], (L-L) = dppe (1,2-bis(diphenylphosphino)ethane), (1), dppp (1,2-bis(diisopropylphosphino)propane), (2), pyNMeP(iPr)2 (N-(diisopropylphosphino)-N-methylpyridin-2-amine), (4), dipe (1,2-bis(diisopropylphosphino)ethane), (5), and [Fe0(L)2(dvtms)], L = PMe3, (3), by a mild reductive route using AlEt2(OEt) as reducing agent. In contrast, by the same methodology, the 18 valence-electron complexes [Fe0(L-L)2(ethylene)], (L-L) = dppm (1,2-bis(diphenylphosphino)methane), 6, (L-L) = dppa (1,2-bis(diphenylphosphino)amine) 7 or (L-L)=dppe, 8, were obtained, which do not contain dvtms. In addition, a combined DFT and solid-state paramagnetic NMR methodology is introduced for the structure determination of 5. A comparative study of the reactivity of 1,2,4-6 and 8 with 3-hexyne highlights emerging mechanistic implications for C-C coupling reactions using these complexes as catalysts.
Conflict of interest statement
The authors declare no competing financial interest.
Figures



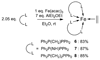


Similar articles
-
Heteroleptic silver(I) complexes prepared from phenanthroline and bis-phosphine ligands.Inorg Chem. 2013 Dec 16;52(24):14343-54. doi: 10.1021/ic402342y. Epub 2013 Nov 27. Inorg Chem. 2013. PMID: 24279392
-
Heteroleptic copper(I) complexes prepared from phenanthroline and bis-phosphine ligands.Inorg Chem. 2013 Oct 21;52(20):12140-51. doi: 10.1021/ic4020042. Epub 2013 Oct 1. Inorg Chem. 2013. PMID: 24083360
-
Phosphine induced migratory CO insertion into the Fe-CH2 bond of the organometallic polymer -[(eta(5)-C5H4)Fe(CO)2CH2SiMe2]n- and characterization of model iron complexes.Dalton Trans. 2010 Aug 14;39(30):7125-31. doi: 10.1039/b925936h. Epub 2010 Jun 4. Dalton Trans. 2010. PMID: 20523957
-
Hexanuclear gold(I) phosphide complexes as platforms for multiple redox-active ferrocenyl units.Chemistry. 2014 Jan 3;20(1):304-10. doi: 10.1002/chem.201301948. Chemistry. 2014. PMID: 24375681
-
Comparison of structure and reactivity of phosphine-amido and hemilabile phosphine-amine chelates of rhodium.Inorg Chem. 2011 Jun 20;50(12):5361-78. doi: 10.1021/ic101883u. Epub 2011 May 17. Inorg Chem. 2011. PMID: 21591636
Cited by
-
Homotropic Cooperativity in Iron-Catalyzed Alkyne Cyclotrimerizations.ACS Catal. 2023 Apr 28;13(10):6610-6618. doi: 10.1021/acscatal.3c00764. eCollection 2023 May 19. ACS Catal. 2023. PMID: 37229435 Free PMC article.
-
Proton-detected fast-magic-angle spinning NMR of paramagnetic inorganic solids.RSC Adv. 2021 Sep 29;11(47):29870-29876. doi: 10.1039/d1ra04110j. eCollection 2021 Sep 1. RSC Adv. 2021. PMID: 35479571 Free PMC article.
References
-
- Bauer I, Knölker H-J. Chem Rev. 2015;115:3170–3387. - PubMed
- Burcher B, Breuil P-AR, Magna L, Olivier-Bourbigou H. In: Iron Catalysis II. Bauer E, editor. Vol. 50. Springer International Publishing; Switzerland: 2015. pp. 217–258.
-
- Lavallo V, El-Batta A, Bertrand G, Grubbs RH. Angew Chem Int Ed. 2011;50:268–271. - PMC - PubMed
- Ung G, Rittle J, Soleilhavoup M, Bertrand G, Peters JC. Angew Chem Int Ed. 2014;53:8427–8431. - PubMed
- Danopoulos AA, Wright JA, Motherwell WB. Chem Comm. 2005:784–786. - PubMed
- Brennessel WW, Jilek RE, Ellis JE. Angew Chem Int Ed. 2007;46:6132–6136. - PubMed
- Werncke CG, Bunting PC, Duhayon C, Long JR, Bontemps S, Sabo-Etienne S. Angew Chem Int Ed. 2015;54:245–248. - PubMed
- Zadrozny JM, Xiao DJ, Atanasov M, Long GJ, Granjean F, Neese F, Long JR. Nature Chem. 2013;5:577–581. - PubMed
-
- Hoyt JM, Schmidt VA, Tondreau AM, Chirik PJ. Science. 2015;349:960–963. - PubMed
- Anderson JS, Rittle J, Peters JC. Nature. 2013;501:84–88. - PMC - PubMed
- Creutz SE, Peters JC. J Am Chem Soc. 2014;136:1105–1115. - PMC - PubMed
- Ung G, Peters JC. Angew Chem Int Ed. 2015;54:532–535. - PMC - PubMed
- Boddien A, Loges B, Gärtner F, Toborg C, Fumino K, Junge H, Ludwig R, Beller M. J Am Chem Soc. 2010;132:8924–8934. - PubMed
- Fürstner A, Majima K, Martín R, Krause H, Kattnig E, Goddard R, Lehmann CW. J Am Chem Soc. 2008;130:1992–2004. - PubMed
- Fürstner A, Martín R, Krause H, Seidel G, Goddard R, Lehmann CW. J Am Chem Soc. 2008;130:8773–8787. - PubMed
- Bart SC, Lobkovsky E, Chirik PJ. J Am Chem Soc. 2004;126:13794–13807. - PubMed
- Wang C, Li X, Wu F, Wan B. Angew Chem Int Ed. 2011;50:7162–7166. - PubMed
-
- McNeill E, Ritter T. Acc Chem Res. 2015;48:2330–2343. - PubMed
-
-
Selected examples of low-oxidation state iron complexes stabilized by nitrogen-based ligands: Stoian SA, Yu Y, Smith JM, Holland PL, Bominaar EL, Münck E. Inorg Chem. 2005;44:4915–4922. Yu Y, Smith JM, Flaschenriem CJ, Holland PL. Inorg Chem. 2006;45:5742–5751. Smith JM, Sadique AR, Cundari TR, Rogers KR, Lukat-Rodgers G, Lachicotte RJ, Flaschenriem CJ, Vela J, Holland PL. J Am Chem Soc. 2006;128:756–769. Chiang KP, Scarborough CC, Horitani M, Lees NS, Ding K, Dugan TR, Brenessel WW, Bill E, Hoffman BM, Holland PL. Angew Chem Int Ed. 2012;51:3658–3662. Rodriguez MM, Stubbert BD, Scarborough CC, Brennessel WW, Bill E, Holland PL. Angew Chem Int Ed. 2012;51:8247–8250. Coric I, Mercado BQ, Bill E, Vinyard DJ, Holland PL. Nature. 2015;526:96–99. Russell SK, Lobkovsky E, Chirik PJJ. Am Chem Soc. 2011;133:8858–8851. Hoyt JM, Sylvester KT, Semproni SP, Chirik PJ. J Am Chem Soc. 2013;135:4862–4877.
-
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous
