The Discovery and Development of Liraglutide and Semaglutide
- PMID: 31031702
- PMCID: PMC6474072
- DOI: 10.3389/fendo.2019.00155
The Discovery and Development of Liraglutide and Semaglutide
Abstract
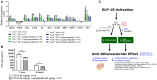
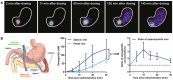
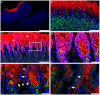
The discovery of glucagon-like peptide-1 (GLP-1), an incretin hormone with important effects on glycemic control and body weight regulation, led to efforts to extend its half-life and make it therapeutically effective in people with type 2 diabetes (T2D). The development of short- and then long-acting GLP-1 receptor agonists (GLP-1RAs) followed. Our article charts the discovery and development of the long-acting GLP-1 analogs liraglutide and, subsequently, semaglutide. We examine the chemistry employed in designing liraglutide and semaglutide, the human and non-human studies used to investigate their cellular targets and pharmacological effects, and ongoing investigations into new applications and formulations of these drugs. Reversible binding to albumin was used for the systemic protraction of liraglutide and semaglutide, with optimal fatty acid and linker combinations identified to maximize albumin binding while maintaining GLP-1 receptor (GLP-1R) potency. GLP-1RAs mediate their effects via this receptor, which is expressed in the pancreas, gastrointestinal tract, heart, lungs, kidneys, and brain. GLP-1Rs in the pancreas and brain have been shown to account for the respective improvements in glycemic control and body weight that are evident with liraglutide and semaglutide. Both liraglutide and semaglutide also positively affect cardiovascular (CV) outcomes in individuals with T2D, although the precise mechanism is still being explored. Significant weight loss, through an effect to reduce energy intake, led to the approval of liraglutide (3.0 mg) for the treatment of obesity, an indication currently under investigation with semaglutide. Other ongoing investigations with semaglutide include the treatment of non-alcoholic fatty liver disease (NASH) and its use in an oral formulation for the treatment of T2D. In summary, rational design has led to the development of two long-acting GLP-1 analogs, liraglutide and semaglutide, that have made a vast contribution to the management of T2D in terms of improvements in glycemic control, body weight, blood pressure, lipids, beta-cell function, and CV outcomes. Furthermore, the development of an oral formulation for semaglutide may provide individuals with additional benefits in relation to treatment adherence. In addition to T2D, liraglutide is used in the treatment of obesity, while semaglutide is currently under investigation for use in obesity and NASH.
Keywords: GLP-1; albumin; liraglutide; obesity; once-weekly; semaglutide; type 2 diabetes.
Figures


References
-
- Nauck MA, Heimesaat MM, Orskov C, Holst JJ, Ebert R, Creutzfeldt W. Preserved incretin activity of glucagon-like peptide 1 [7-36 amide] but not of synthetic human gastric inhibitory polypeptide in patients with type-2 diabetes mellitus. J Clin Invest. (1993) 91:301–7. 10.1172/JCI116186 - DOI - PMC - PubMed
-
- Deacon CF, Nauck MA, Toft-Nielsen M, Pridal L, Willms B, Holst JJ. Both subcutaneously and intravenously administered glucagon-like peptide I are rapidly degraded from the NH2-terminus in type II diabetic patients and in healthy subjects. Diabetes. (1995) 44:1126–31. 10.2337/diab.44.9.1126 - DOI - PubMed
Publication types
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

