Membrane Sphingolipids Regulate the Fitness and Antifungal Protein Susceptibility of Neurospora crassa
- PMID: 31031714
- PMCID: PMC6471014
- DOI: 10.3389/fmicb.2019.00605
Membrane Sphingolipids Regulate the Fitness and Antifungal Protein Susceptibility of Neurospora crassa
Abstract
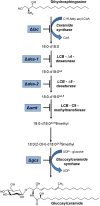
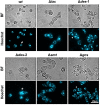
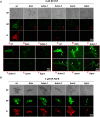
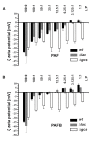
The membrane sphingolipid glucosylceramide (GlcCer) plays an important role in fungal fitness and adaptation to most diverse environments. Moreover, reported differences in the structure of GlcCer between fungi, plants and animals render this pathway a promising target for new generation therapeutics. Our knowledge about the GlcCer biosynthesis in fungi is mainly based on investigations of yeasts, whereas this pathway is less well characterized in molds. We therefore performed a detailed lipidomic profiling of GlcCer species present in Neurospora crassa and comprehensively show that the deletion of genes encoding enzymes involved in GlcCer biosynthesis affects growth, conidiation and stress response in this model fungus. Importantly, our study evidences that differences in the pathway intermediates and their functional role exist between N. crassa and other fungal species. We further investigated the role of GlcCer in the susceptibility of N. crassa toward two small cysteine-rich and cationic antimicrobial proteins (AMPs), PAF and PAFB, which originate from the filamentous ascomycete Penicillium chrysogenum. The interaction of these AMPs with the fungal plasma membrane is crucial for their antifungal toxicity. We found that GlcCer determines the susceptibility of N. crassa toward PAF, but not PAFB. A higher electrostatic affinity of PAFB than PAF to anionic membrane surfaces might explain the difference in their antifungal mode of action.
Keywords: Neurospora crassa; Penicillium chrysogenum; antimicrobial proteins; glucosylceramide; lipidomics; sphingolipids.
Figures





Similar articles
-
Two small, cysteine-rich and cationic antifungal proteins from Penicillium chrysogenum: A comparative study of PAF and PAFB.Biochim Biophys Acta Biomembr. 2020 Aug 1;1862(8):183246. doi: 10.1016/j.bbamem.2020.183246. Epub 2020 Mar 3. Biochim Biophys Acta Biomembr. 2020. PMID: 32142818 Free PMC article. Review.
-
The antifungal activity of the Penicillium chrysogenum protein PAF disrupts calcium homeostasis in Neurospora crassa.Eukaryot Cell. 2010 Sep;9(9):1374-82. doi: 10.1128/EC.00050-10. Epub 2010 Jul 9. Eukaryot Cell. 2010. PMID: 20622001 Free PMC article.
-
Nutrient Excess Triggers the Expression of the Penicillium chrysogenum Antifungal Protein PAFB.Microorganisms. 2019 Dec 4;7(12):654. doi: 10.3390/microorganisms7120654. Microorganisms. 2019. PMID: 31817241 Free PMC article.
-
New Antimicrobial Potential and Structural Properties of PAFB: A Cationic, Cysteine-Rich Protein from Penicillium chrysogenum Q176.Sci Rep. 2018 Jan 29;8(1):1751. doi: 10.1038/s41598-018-20002-2. Sci Rep. 2018. PMID: 29379111 Free PMC article.
-
The Penicillium chrysogenum antifungal protein PAF, a promising tool for the development of new antifungal therapies and fungal cell biology studies.Cell Mol Life Sci. 2008 Feb;65(3):445-54. doi: 10.1007/s00018-007-7364-8. Cell Mol Life Sci. 2008. PMID: 17965829 Free PMC article. Review.
Cited by
-
Two small, cysteine-rich and cationic antifungal proteins from Penicillium chrysogenum: A comparative study of PAF and PAFB.Biochim Biophys Acta Biomembr. 2020 Aug 1;1862(8):183246. doi: 10.1016/j.bbamem.2020.183246. Epub 2020 Mar 3. Biochim Biophys Acta Biomembr. 2020. PMID: 32142818 Free PMC article. Review.
-
Navigating the fungal battlefield: cysteine-rich antifungal proteins and peptides from Eurotiales.Front Fungal Biol. 2024 Sep 3;5:1451455. doi: 10.3389/ffunb.2024.1451455. eCollection 2024. Front Fungal Biol. 2024. PMID: 39323611 Free PMC article. Review.
-
Membrane-Interacting Antifungal Peptides.Front Cell Dev Biol. 2021 Apr 12;9:649875. doi: 10.3389/fcell.2021.649875. eCollection 2021. Front Cell Dev Biol. 2021. PMID: 33912564 Free PMC article. Review.
-
Sterol-Response Pathways Mediate Alkaline Survival in Diverse Fungi.mBio. 2020 Jun 16;11(3):e00719-20. doi: 10.1128/mBio.00719-20. mBio. 2020. PMID: 32546619 Free PMC article.
-
Species-Specific Differences in the Susceptibility of Fungi to the Antifungal Protein AFP Depend on C-3 Saturation of Glycosylceramides.mSphere. 2019 Dec 11;4(6):e00741-19. doi: 10.1128/mSphere.00741-19. mSphere. 2019. PMID: 31826973 Free PMC article.
References
Grants and funding
LinkOut - more resources
Full Text Sources

