Targeted NGS Platforms for Genetic Screening and Gene Discovery in Primary Immunodeficiencies
- PMID: 31031743
- PMCID: PMC6470723
- DOI: 10.3389/fimmu.2019.00316
Targeted NGS Platforms for Genetic Screening and Gene Discovery in Primary Immunodeficiencies
Erratum in
-
Corrigendum: Targeted NGS Platforms for Genetic Screening and Gene Discovery in Primary Immunodeficiencies.Front Immunol. 2019 May 31;10:1184. doi: 10.3389/fimmu.2019.01184. eCollection 2019. Front Immunol. 2019. PMID: 31214169 Free PMC article.
Abstract
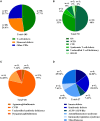
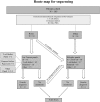
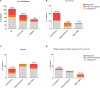
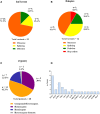
Background: Primary Immunodeficiencies (PIDs) are a heterogeneous group of genetic immune disorders. While some PIDs can manifest with more than one phenotype, signs, and symptoms of various PIDs overlap considerably. Recently, novel defects in immune-related genes and additional variants in previously reported genes responsible for PIDs have been successfully identified by Next Generation Sequencing (NGS), allowing the recognition of a broad spectrum of disorders. Objective: To evaluate the strength and weakness of targeted NGS sequencing using custom-made Ion Torrent and Haloplex (Agilent) panels for diagnostics and research purposes. Methods: Five different panels including known and candidate genes were used to screen 105 patients with distinct PID features divided in three main PID categories: T cell defects, Humoral defects and Other PIDs. The Ion Torrent sequencing platform was used in 73 patients. Among these, 18 selected patients without a molecular diagnosis and 32 additional patients were analyzed by Haloplex enrichment technology. Results: The complementary use of the two custom-made targeted sequencing approaches allowed the identification of causative variants in 28.6% (n = 30) of patients. Twenty-two out of 73 (34.6%) patients were diagnosed by Ion Torrent. In this group 20 were included in the SCID/CID category. Eight out of 50 (16%) patients were diagnosed by Haloplex workflow. Ion Torrent method was highly successful for those cases with well-defined phenotypes for immunological and clinical presentation. The Haloplex approach was able to diagnose 4 SCID/CID patients and 4 additional patients with complex and extended phenotypes, embracing all three PID categories in which this approach was more efficient. Both technologies showed good gene coverage. Conclusions: NGS technology represents a powerful approach in the complex field of rare disorders but its different application should be weighted. A relatively small NGS target panel can be successfully applied for a robust diagnostic suspicion, while when the spectrum of clinical phenotypes overlaps more than one PID an in-depth NGS analysis is required, including also whole exome/genome sequencing to identify the causative gene.
Keywords: Haloplex; Ion Torrent; Next Generation Sequencing; gene panels; primary immunodeficiencies.
Figures
References
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources

