Autophagy Limits Inflammasome During Chlamydia pneumoniae Infection
- PMID: 31031755
- PMCID: PMC6473188
- DOI: 10.3389/fimmu.2019.00754
Autophagy Limits Inflammasome During Chlamydia pneumoniae Infection
Abstract
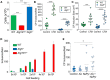
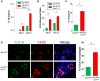
Autophagy can either antagonize or promote intracellular bacterial growth, depending on the pathogen. Here, we investigated the role of autophagy during a pulmonary infection with the obligate intracellular pathogen, Chlamydia pneumoniae (CP). In mouse embryonic fibroblasts (MEFs) or macrophages, deficiency of autophagy pathway components led to enhanced CP replication, suggesting that autophagy exerts a bactericidal role. However, in vivo, mice with myeloid-specific deletion of the autophagic protein ATG16L1 suffered increased mortality during CP infection, neutrophilia, and increased inflammasome activation despite no change in bacterial burden. Induction of autophagy led to reduced CP replication in vitro, but impaired survival in CP-infected mice, associated with an initial reduction in IL-1β production, followed by enhanced neutrophil recruitment, defective CP clearance, and later inflammasome activation and IL-1β production, which drove the resulting mortality. Taken together, our data suggest that a delicate interplay exists between autophagy and inflammasome activation in determining the outcome of CP infection, perturbation of which can result in inflammatory pathology or unrestricted bacterial growth.
Keywords: Chlamydia pneumoniae; IL-1β; autophagy; inflammasome; macrophages.
Figures



Similar articles
-
Chlamydia pneumoniae harness host NLRP3 inflammasome-mediated caspase-1 activation for optimal intracellular growth in murine macrophages.Biochem Biophys Res Commun. 2014 Sep 26;452(3):689-94. doi: 10.1016/j.bbrc.2014.08.128. Epub 2014 Sep 1. Biochem Biophys Res Commun. 2014. PMID: 25193701
-
Synergistic Costimulatory Effect of Chlamydia pneumoniae with Carbon Nanoparticles on NLRP3 Inflammasome-Mediated Interleukin-1β Secretion in Macrophages.Infect Immun. 2015 Jul;83(7):2917-25. doi: 10.1128/IAI.02968-14. Epub 2015 May 4. Infect Immun. 2015. PMID: 25939513 Free PMC article.
-
Deficiency of XIAP leads to sensitization for Chlamydophila pneumoniae pulmonary infection and dysregulation of innate immune response in mice.J Biol Chem. 2010 Jun 25;285(26):20291-302. doi: 10.1074/jbc.M109.096297. Epub 2010 Apr 28. J Biol Chem. 2010. PMID: 20427267 Free PMC article.
-
Murine Gammaherpesvirus 68 Pathogenesis Is Independent of Caspase-1 and Caspase-11 in Mice and Impairs Interleukin-1β Production upon Extrinsic Stimulation in Culture.J Virol. 2015 Jul;89(13):6562-74. doi: 10.1128/JVI.00658-15. Epub 2015 Apr 8. J Virol. 2015. PMID: 25855746 Free PMC article.
-
Chlamydia pneumoniae and atherosclerosis.Semin Respir Infect. 2003 Mar;18(1):48-54. doi: 10.1053/srin.2003.50006. Semin Respir Infect. 2003. PMID: 12652454 Review.
Cited by
-
ROS-AMPK/mTOR-dependent enterocyte autophagy is involved in the regulation of Giardia infection-related tight junction protein and nitric oxide levels.Front Immunol. 2023 Mar 14;14:1120996. doi: 10.3389/fimmu.2023.1120996. eCollection 2023. Front Immunol. 2023. PMID: 36999034 Free PMC article.
-
Interactions of Autophagy and the Immune System in Health and Diseases.Autophagy Rep. 2022;1(1):438-515. doi: 10.1080/27694127.2022.2119743. Epub 2022 Oct 5. Autophagy Rep. 2022. PMID: 37425656 Free PMC article.
-
Autophagy: the misty lands of Chlamydia trachomatis infection.Front Cell Infect Microbiol. 2024 Sep 6;14:1442995. doi: 10.3389/fcimb.2024.1442995. eCollection 2024. Front Cell Infect Microbiol. 2024. PMID: 39310786 Free PMC article. Review.
-
Salmonella spvC Gene Inhibits Autophagy of Host Cells and Suppresses NLRP3 as Well as NLRC4.Front Immunol. 2021 Jul 14;12:639019. doi: 10.3389/fimmu.2021.639019. eCollection 2021. Front Immunol. 2021. PMID: 34335562 Free PMC article.
-
Chlamydia pneumoniae in Alzheimer's disease pathology.Front Neurosci. 2024 May 6;18:1393293. doi: 10.3389/fnins.2024.1393293. eCollection 2024. Front Neurosci. 2024. PMID: 38770241 Free PMC article. Review.
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

