Diagnostic Biomarkers to Diagnose Acute Allograft Rejection After Liver Transplantation: Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies
- PMID: 31031758
- PMCID: PMC6470197
- DOI: 10.3389/fimmu.2019.00758
Diagnostic Biomarkers to Diagnose Acute Allograft Rejection After Liver Transplantation: Systematic Review and Meta-Analysis of Diagnostic Accuracy Studies
Abstract
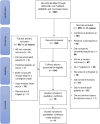
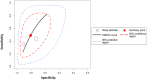
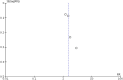
Objective: A systematic review and meta-analysis of diagnostic biomarkers for noninvasive diagnosis of acute allograft rejection following liver transplantation. Background: Noninvasive blood and urine markers have been widely explored in recent decades for diagnosing acute rejection after liver transplantation. However, none have been translated into routine clinical use so far due to uncertain diagnostic accuracy, and liver biopsy remains the gold standard. Methods: Systematic literature searches of Medline, Cochrane and Embase were conducted up to February 2019 to identify studies evaluating the use of noninvasive markers in diagnosing allograft rejection following liver transplantation. Meta-analysis was performed using a random effects model with DerSimonian-Laird weighting and the hierarchical summary receiver operating curve. Results: Of 560 identified studies, 15 studies (1,445 patients) met the inclusion criteria. The following markers were tested: acid labile nitroso-compounds (NOx), serum amyloid A protein, procalcitonin, peripheral blood eosinophil count, peripheral blood T-cell activation and interleukin 2 (IL-2) receptor, guanylate-binding protein-2 mRNA, graft-derived cell-free DNA, pi-glutathione S-transferase, alpha-glutathione S-transferase and serum HLA class I soluble antigens. Only eosinophil count was tested in multiple studies, and they demonstrated high heterogeneity (I2 = 72% [95% CI: 0.5-0.99]). IL-2 receptor demonstrated the highest sensitivity (89% [95% CI: 0.78-0.96]) and specificity (81% [95% CI: 0.69-0.89]). Conclusion: IL-2 receptor expression demonstrated the highest diagnostic accuracy, while the peripheral eosinophil count was the only marker tested in more than one study. Presently, liver biopsy remains superior to noninvasive diagnostic biomarkers as most studies exhibited inferior designs, hindering possible translation into clinical application.
Keywords: acute rejection; diagnostic accuracy; diagnostic biomarker; liver transplantation; non-invasive test.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Research Materials
Miscellaneous

