Bioproduction of a Therapeutic Vaccine Against Human Papillomavirus in Tomato Hairy Root Cultures
- PMID: 31031788
- PMCID: PMC6470201
- DOI: 10.3389/fpls.2019.00452
Bioproduction of a Therapeutic Vaccine Against Human Papillomavirus in Tomato Hairy Root Cultures
Abstract
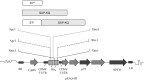
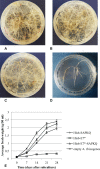
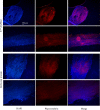
Human papillomavirus (HPV) tumor disease is a critical public health problem worldwide, especially in the developing countries. The recognized pathogenic function of E5, E6, and E7 oncoproteins offers the opportunity to devise therapeutic vaccines based on engineered recombinant proteins. The potential of plants to manufacture engineered compounds for pharmaceutical purposes, from small to complex protein molecules, allows the expression of HPV antigens and, possibly, the regulation of immune functions to develop very specific therapies as a reinforcement to available nonspecific therapies and preventive vaccination also in developed countries. Among plant-based expression formats, hairy root cultures are a robust platform combining the benefits of eukaryotic plant-based bioreactors, with those typical of cell cultures. In this work, to devise an experimental therapeutic vaccine against HPV, hairy root cultures were used to express a harmless form of the HPV type 16 E7 protein (E7*) fused to SAPKQ, a noncytotoxic form of the saporin protein from Saponaria officinalis, that we had shown to improve E7-specific cell-mediated responses as a fusion E7*-SAPKQ DNA vaccine. Hairy root clones expressing the E7*-SAPKQ candidate vaccine were obtained upon infection of leaf explants of Solanum lycopersicum using a recombinant plant expression vector. Yield was approximately 35.5 μg/g of fresh weight. Mouse immunization with vaccine-containing crude extracts was performed together with immunological and biological tests to investigate immune responses and anticancer activity, respectively. Animals were primed with either E7*-SAPKQ DNA-based vaccine or E7*-SAPKQ root extract-based vaccine and boosted with the same (homologous schedule) or with the other vaccine preparation (heterologous schedule) in the context of TC-1 experimental mouse model of HPV-associated tumor. All the formulations exhibited an immunological response associated to anticancer activity. In particular, DNA as prime and hairy root extract as boost demonstrated the highest efficacy. This work, based on the development of low-cost technologies, highlights the suitability of hairy root cultures as possible biofactories of therapeutic HPV vaccines and underlines the importance of the synergic combination of treatment modalities for future developments in this field.
Keywords: HPV – human papillomavirus; cancer; hairy root cultures; heterologous prime – boost; plant molecular farming; plant-produced antigens; therapeutic vaccines.
Figures





Similar articles
-
Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers.Cancers (Basel). 2020 Oct 23;12(11):3101. doi: 10.3390/cancers12113101. Cancers (Basel). 2020. PMID: 33114220 Free PMC article. Review.
-
Optimization of heterologous DNA-prime, protein boost regimens and site of vaccination to enhance therapeutic immunity against human papillomavirus-associated disease.Cell Biosci. 2016 Feb 25;6:16. doi: 10.1186/s13578-016-0080-z. eCollection 2016. Cell Biosci. 2016. PMID: 26918115 Free PMC article.
-
Prime-boost therapeutic vaccination in mice with DNA/DNA or DNA/Fowlpox virus recombinants expressing the Human Papilloma Virus type 16 E6 and E7 mutated proteins fused to the coat protein of Potato virus X.Virus Res. 2016 Oct 2;225:82-90. doi: 10.1016/j.virusres.2016.09.011. Epub 2016 Sep 21. Virus Res. 2016. PMID: 27664839
-
Optimal induction of HPV DNA vaccine-induced CD8+ T cell responses and therapeutic antitumor effect by antigen engineering and electroporation.Vaccine. 2009 Sep 25;27(42):5906-12. doi: 10.1016/j.vaccine.2009.07.033. Epub 2009 Aug 3. Vaccine. 2009. PMID: 19651174
-
The Efficacy of Therapeutic DNA Vaccines Expressing the Human Papillomavirus E6 and E7 Oncoproteins for Treatment of Cervical Cancer: Systematic Review.Vaccines (Basel). 2021 Dec 31;10(1):53. doi: 10.3390/vaccines10010053. Vaccines (Basel). 2021. PMID: 35062714 Free PMC article. Review.
Cited by
-
Nicotiana hairy roots for recombinant protein expression, where to start? A systematic review.Mol Biol Rep. 2023 May;50(5):4587-4604. doi: 10.1007/s11033-023-08360-1. Epub 2023 Mar 14. Mol Biol Rep. 2023. PMID: 36917368
-
Hairy root culture: a potent method for improved secondary metabolite production of Solanaceous plants.Front Plant Sci. 2023 Sep 4;14:1197555. doi: 10.3389/fpls.2023.1197555. eCollection 2023. Front Plant Sci. 2023. PMID: 37731987 Free PMC article. Review.
-
Plant-Derived Natural Compounds in Genetic Vaccination and Therapy for HPV-Associated Cancers.Cancers (Basel). 2020 Oct 23;12(11):3101. doi: 10.3390/cancers12113101. Cancers (Basel). 2020. PMID: 33114220 Free PMC article. Review.
-
Collection of Hairy Roots as a Basis for Fundamental and Applied Research.Molecules. 2022 Nov 19;27(22):8040. doi: 10.3390/molecules27228040. Molecules. 2022. PMID: 36432139 Free PMC article. Review.
-
Immunotherapeutic effects of recombinant colorectal cancer antigen produced in tomato fruits.Sci Rep. 2022 Jun 13;12(1):9723. doi: 10.1038/s41598-022-13839-1. Sci Rep. 2022. PMID: 35697846 Free PMC article.
References
LinkOut - more resources
Full Text Sources

