Mitochondrial DNA Damage Does Not Determine C. elegans Lifespan
- PMID: 31031801
- PMCID: PMC6473201
- DOI: 10.3389/fgene.2019.00311
Mitochondrial DNA Damage Does Not Determine C. elegans Lifespan
Abstract
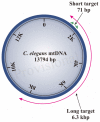
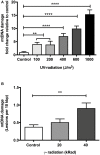
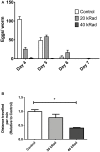
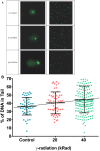
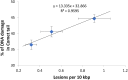
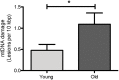
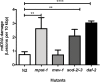
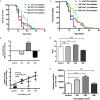
The mitochondrial free radical theory of aging (mFRTA) proposes that accumulation of oxidative damage to macromolecules in mitochondria is a causative mechanism for aging. Accumulation of mitochondrial DNA (mtDNA) damage may be of particular interest in this context. While there is evidence for age-dependent accumulation of mtDNA damage, there have been only a limited number of investigations into mtDNA damage as a determinant of longevity. This lack of quantitative data regarding mtDNA damage is predominantly due to a lack of reliable assays to measure mtDNA damage. Here, we report adaptation of a quantitative real-time polymerase chain reaction (qRT-PCR) assay for the detection of sequence-specific mtDNA damage in C. elegans and apply this method to investigate the role of mtDNA damage in the aging of nematodes. We compare damage levels in old and young animals and also between wild-type animals and long-lived mutant strains or strains with modifications in ROS detoxification or production rates. We confirm an age-dependent increase in mtDNA damage levels in C. elegans but found that there is no simple relationship between mtDNA damage and lifespan. MtDNA damage levels were high in some mutants with long lifespan (and vice versa). We next investigated mtDNA damage, lifespan and healthspan effects in nematode subjected to exogenously elevated damage (UV- or γ-radiation induced). We, again, observed a complex relationship between damage and lifespan in such animals. Despite causing a significant elevation in mtDNA damage, γ-radiation did not shorten the lifespan of nematodes at any of the doses tested. When mtDNA damage levels were elevated significantly using UV-radiation, nematodes did suffer from shorter lifespan at the higher end of exposure tested. However, surprisingly, we also found hormetic lifespan and healthspan benefits in nematodes treated with intermediate doses of UV-radiation, despite the fact that mtDNA damage in these animals was also significantly elevated. Our results suggest that within a wide physiological range, the level of mtDNA damage does not control lifespan in C. elegans.
Keywords: DNA damage; aging; healthspan; hormesis; lifespan; mitochondrial DNA; quantitative PCR; radiation.
Figures
Similar articles
-
Mitochondrial changes in ageing Caenorhabditis elegans--what do we learn from superoxide dismutase knockouts?PLoS One. 2011;6(5):e19444. doi: 10.1371/journal.pone.0019444. Epub 2011 May 18. PLoS One. 2011. PMID: 21611128 Free PMC article.
-
The mitochondrial free radical theory of aging: a critical view.Curr Aging Sci. 2008 Mar;1(1):10-21. doi: 10.2174/1874609810801010010. Curr Aging Sci. 2008. PMID: 20021368
-
Regulation of longevity and oxidative stress by nutritional interventions: role of methionine restriction.Exp Gerontol. 2013 Oct;48(10):1030-42. doi: 10.1016/j.exger.2013.02.021. Epub 2013 Feb 27. Exp Gerontol. 2013. PMID: 23454735 Review.
-
Mitochondrial stress extends lifespan in C. elegans through neuronal hormesis.Exp Gerontol. 2014 Aug;56:89-98. doi: 10.1016/j.exger.2014.03.026. Epub 2014 Apr 4. Exp Gerontol. 2014. PMID: 24709340
-
Reactive Oxygen Species and Aging in Caenorhabditis elegans: Causal or Casual Relationship?Antioxid Redox Signal. 2010 Dec 15;13(12):1911-53. doi: 10.1089/ars.2010.3215. Epub 2010 Sep 13. Antioxid Redox Signal. 2010. PMID: 20568954 Review.
Cited by
-
Aging through the lens of mitochondrial DNA mutations and inheritance paradoxes.Biogerontology. 2024 Dec 27;26(1):33. doi: 10.1007/s10522-024-10175-x. Biogerontology. 2024. PMID: 39729246 Review.
-
Mitochondrial mutations in Caenorhabditis elegans show signatures of oxidative damage and an AT-bias.Genetics. 2021 Oct 2;219(2):iyab116. doi: 10.1093/genetics/iyab116. Genetics. 2021. PMID: 34849888 Free PMC article.
-
Aging: All roads lead to mitochondria.Semin Cell Dev Biol. 2021 Aug;116:160-168. doi: 10.1016/j.semcdb.2021.02.006. Epub 2021 Mar 23. Semin Cell Dev Biol. 2021. PMID: 33741252 Free PMC article.
-
Role of the mtDNA Mutations and Mitophagy in Inflammaging.Int J Mol Sci. 2022 Jan 25;23(3):1323. doi: 10.3390/ijms23031323. Int J Mol Sci. 2022. PMID: 35163247 Free PMC article. Review.
-
Changes in the Mitochondria in the Aging Process-Can α-Tocopherol Affect Them?Int J Mol Sci. 2023 Aug 5;24(15):12453. doi: 10.3390/ijms241512453. Int J Mol Sci. 2023. PMID: 37569829 Free PMC article. Review.
References
LinkOut - more resources
Full Text Sources
Other Literature Sources

