Time to All-cause Treatment Discontinuation as the Primary Outcome in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Study
- PMID: 31031886
- PMCID: PMC6482974
- DOI: 10.1198/sbr.2011.10013
Time to All-cause Treatment Discontinuation as the Primary Outcome in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) Schizophrenia Study
Abstract
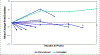
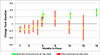
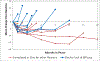
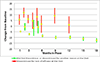
Time until all-cause treatment discontinuation was the primary outcome of the CATIE trial. We discuss the advantages and disadvantages of this outcome, and evaluate its association with clinical correlates through graphical response profiles. We investigate the characteristics of patients who discontinued for patient decision, including a reclassification of patient decision into other reasons. All-cause discontinuation is compared to a related outcome, time until treatment failure. Patients who discontinued had lower quality of life scores than other patients. Patients discontinuing for lack of efficacy had worsened efficacy scores compared with an improvement for other patients. Those who discontinued for patient decision had lower compliance. Blinded reclassification of discontinuation for patient decision identified 5% of cases as lack of efficacy and 21% as intolerable side effects. Reclassified patients participated in the next study phase at a higher rate than those remaining as patient decision (67% vs. 10%). Treatment group differences for time to discontinuation due to patient decision were attenuated after censoring the reclassified patients, but were still suggestive. Treatment comparisons for time to treatment failure were consistent with all cause discontinuation, although somewhat smaller. All-cause discontinuation is recommended as a simple and comprehensive outcome for pharmaceutical Phase II-IV clinical trials.
Keywords: drop-out; graphical response profiles; informative missing data; lost to follow-up; treatment failure.
Figures
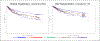
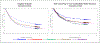
References
-
- Alison P (2010), “Survival Analysis Using the SAS® System: A Practical Guide, Second Edition” Cary, N.C., SAS Institute, Inc.
-
- Cheng Y, Fine JP, Kosorok MR (2007), “Nonparametric Association Analysis of Bivariate Competing Risks Data,” Journal of American Statistical Association, 102(480), 1407–1415.
-
- Cox D (1972), “Regression models and Life Tables,” Journal of the Royal Statistical Society, B34, 187–220.
-
- Davis SM, Koch GG, Davis CE, and LaVange LM (2003), “ Statistical approaches to effectiveness measurement and outcome-driven re-randomizations in the Clinical Antipsychotic Trials of Intervention Effectiveness (CATIE) studies,” Schizophrenia Bulletin, 29(1), 73–80. - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources
