Characterization of Functional Effects of Two New Active Fractions Isolated From Scorpion Venom on Neuronal Ca2+ Spikes: A Possible Action on Ca2+-Dependent Dependent K+ Channels
- PMID: 31031893
- PMCID: PMC6484188
- DOI: 10.32598/bcn.9.10.350
Characterization of Functional Effects of Two New Active Fractions Isolated From Scorpion Venom on Neuronal Ca2+ Spikes: A Possible Action on Ca2+-Dependent Dependent K+ Channels
Abstract
Introduction: It is a long time that natural toxin research is conducted to unlock the medical potential of toxins. Although venoms-toxins cause pathophysiological conditions, they may be effective to treat several diseases. Since toxins including scorpion toxins target voltage-gated ion channels, they may have profound effects on excitable cells. Therefore, elucidating the cellular and electrophysiological impacts of toxins, particularly scorpion toxins would be helpful in future drug development opportunities.
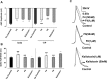
Methods: Intracellular recording was made from F1 cells of Helix aspersa in the presence of calcium Ringer solution in which Na+ and K+ channels were blocked. Then, the modulation of channel function in the presence of extracellular application of F4 and F6 toxins and kaliotoxin (KTX; 50 nM and 1 μM) was examined by assessing the electrophysiological characteristics of calcium spikes.
Results: The two active toxin fractions, similar to KTX, a known Ca2+-activated K+ channel blocker, reduced the amplitude of AHP, enhanced the firing frequency of calcium spikes and broadened the duration of Ca2+ spikes. Therefore, it might be inferred that these two new fractions induce neuronal hyperexcitability possibly, in part, by blocking calcium-activated potassium channel current. However, this supposition requires further investigation using voltage clamping technique.
Conclusion: These toxin fractions may act as blocker of calcium-activated potassium channels.
Keywords: Buthotus schach; Calcium spike; Intracellular recording; Scorpion Venom.
Conflict of interest statement
Conflict of interest The authors declare no conflict of interest.
Figures

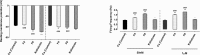
Similar articles
-
Kaliotoxin, a novel peptidyl inhibitor of neuronal BK-type Ca(2+)-activated K+ channels characterized from Androctonus mauretanicus mauretanicus venom.J Biol Chem. 1992 Jan 25;267(3):1640-7. J Biol Chem. 1992. PMID: 1730708
-
Characterization of the outer pore region of the apamin-sensitive Ca2+-activated K+ channel rSK2.Toxicon. 2004 Jun 15;43(8):951-60. doi: 10.1016/j.toxicon.2004.03.025. Toxicon. 2004. PMID: 15208028
-
Structure-activity studies on scorpion toxins that block potassium channels.Toxicon. 1995 Apr;33(4):425-36. doi: 10.1016/0041-0101(94)00181-7. Toxicon. 1995. PMID: 7570628
-
Voltage-Gated K+/Na+ Channels and Scorpion Venom Toxins in Cancer.Front Pharmacol. 2020 Jun 18;11:913. doi: 10.3389/fphar.2020.00913. eCollection 2020. Front Pharmacol. 2020. PMID: 32655396 Free PMC article. Review.
-
Electrophysiological evaluation of the effect of peptide toxins on voltage-gated ion channels: a scoping review on theoretical and methodological aspects with focus on the Central and South American experience.J Venom Anim Toxins Incl Trop Dis. 2024 Sep 2;30:e20230048. doi: 10.1590/1678-9199-JVATITD-2023-0048. eCollection 2024. J Venom Anim Toxins Incl Trop Dis. 2024. PMID: 39263598 Free PMC article.
References
-
- Aboutorabi A., Naderi N., Gholamipourbadie H., Zolfagharian H., Vatanpour H. (2016). Voltage-gated sodium channels modulation by bothutous schach scorpion venom. Iranian Journal of Pharmaceutical Sciences, 12(3), 55–64. [DOI:10.22034/ijps.2016.23841] - DOI
-
- Bal R., Janahmadi M., Green G. G. R., Sanders D. J. (2000). Effect of calcium and calcium channel blockers on transient outward current of F76 and D1 neuronal soma membranes in the subesophageal ganglia of helix aspersa. Journal of Membrane Biology, 173(3), 179–85. [DOI:10.1007/s002320001018] [PMID] - DOI - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous
