A ctenophore (comb jelly) employs vortex rebound dynamics and outperforms other gelatinous swimmers
- PMID: 31032019
- PMCID: PMC6458386
- DOI: 10.1098/rsos.181615
A ctenophore (comb jelly) employs vortex rebound dynamics and outperforms other gelatinous swimmers
Abstract
Gelatinous zooplankton exhibit a wide range of propulsive swimming modes. One of the most energetically efficient is the rowing behaviour exhibited by many species of schyphomedusae, which employ vortex interactions to achieve this result. Ctenophores (comb jellies) typically use a slow swimming, cilia-based mode of propulsion. However, species within the genus Ocyropsis have developed an additional propulsive strategy of rowing the lobes, which are normally used for feeding, in order to rapidly escape from predators. In this study, we used high-speed digital particle image velocimetry to examine the kinematics and fluid dynamics of this rarely studied propulsive mechanism. This mechanism allows Ocyropsis to achieve size-adjusted speeds that are nearly double those of other large gelatinous swimmers. The investigation of the fluid dynamic basis of this escape mode reveals novel vortex interactions that have not previously been described for other biological propulsion systems. The arrangement of vortices during escape swimming produces a similar configuration and impact as that of the well-studied 'vortex rebound' phenomenon which occurs when a vortex ring approaches a solid wall. These results extend our understanding of how animals use vortex-vortex interactions and provide important insights that can inform the bioinspired engineering of propulsion systems.
Keywords: bioengineering; biomechanics; jellyfish; plankton; propulsion; vortex interactions.
Conflict of interest statement
We declare we have no competing interests.
Figures


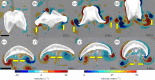

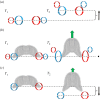
References
-
- Mayor AG. 1912. Ctenophores of the Atlantic coast of North America. Washington, DC: Carnegie Institution of Washington.
-
- Matsumoto G, Harbison G. 1993. In situ observations of foraging, feeding, and escape behavior in three orders of oceanic ctenophores: Lobata, Cestida, and Beroida. Mar. Biol. 117, 279–287. (10.1007/BF00345673) - DOI
-
- Larson R. 1987. Costs of transport for the scyphomedusa Stomolophus meleagris L. Agassiz. Can. J. Zool. 65, 2690–2695. (10.1139/z87-408) - DOI
-
- Costello JH, Colin SP, Dabiri JO. 2008. Medusan morphospace: phylogenetic constraints, biomechanical solutions, and ecological consequences. Invertebr. Biol. 127, 265–290. (10.1111/j.1744-7410.2008.00126.x) - DOI
LinkOut - more resources
Full Text Sources

