Absence of Nonclassical Monocytes in Hemolytic Patients: Free Hb and NO-Mediated Mechanism
- PMID: 31032371
- PMCID: PMC6458887
- DOI: 10.1155/2019/1409383
Absence of Nonclassical Monocytes in Hemolytic Patients: Free Hb and NO-Mediated Mechanism
Abstract
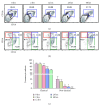
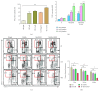
In a recent work, we have described the kinetics among the monocyte subsets in the peripheral blood of hemolytic patients including paroxysmal nocturnal hemoglobinuria (PNH) and sickle cell disease (SCD). After engulfing Hb-activated platelets, classical monocytes (CD14+CD16-) significantly transformed into highly inflammatory (CD14+CD16hi) subsets in vitro. An estimated 40% of total circulating monocytes in PNH and 70% in SCD patients existed as CD14+CD16hi subsets. In this study, we show that the nonclassical (CD14dimCD16+) monocyte subsets are nearly absent in patients with PNH or SCD, compared to 10-12% cells in healthy individuals. In mechanism, we have described the unique role of both free Hb and nitric oxide (NO) in reducing number of nonclassical subsets more than classical monocytes. After engulfing Hb-activated platelets, the monocytes including nonclassical subsets acquired rapid cell death within 12 h in vitro. Further, the treatment to monocytes either with the secretome of Hb-activated platelets containing NO and free Hb or purified free Hb along with GSNO (a physiological NO donor) enhanced rapid cell death. Besides, our data from both PNH and SCD patients exhibited a direct correlation between intracellular NO and cell death marker 7AAD in monocytes from the peripheral blood. Our data together suggest that due to the immune surveillance nature, the nonclassical or patrolling monocytes are encountered frequently by Hb-activated platelets, free Hb, and NO in the circulation of hemolytic patients and are predisposed to die rapidly.
Figures



Similar articles
-
Development of pro-inflammatory phenotype in monocytes after engulfing Hb-activated platelets in hemolytic disorders.Clin Immunol. 2017 Feb;175:133-142. doi: 10.1016/j.clim.2016.12.007. Epub 2016 Dec 28. Clin Immunol. 2017. PMID: 28039017
-
Engulfment of Hb-activated platelets differentiates monocytes into pro-inflammatory macrophages in PNH patients.Eur J Immunol. 2018 Aug;48(8):1285-1294. doi: 10.1002/eji.201747449. Epub 2018 May 17. Eur J Immunol. 2018. PMID: 29677388
-
Neutrophils develop rapid proinflammatory response after engulfing Hb-activated platelets under intravascular hemolysis.Clin Exp Immunol. 2019 Aug;197(2):131-140. doi: 10.1111/cei.13310. Epub 2019 May 27. Clin Exp Immunol. 2019. PMID: 31099890 Free PMC article.
-
[Pathophysiological consequences of hemolysis. Role of cell-free hemoglobin].Postepy Hig Med Dosw (Online). 2011 Sep 28;65:627-39. doi: 10.5604/17322693.961007. Postepy Hig Med Dosw (Online). 2011. PMID: 22100795 Review. Polish.
-
Nonclassical patrolling monocyte function in the vasculature.Arterioscler Thromb Vasc Biol. 2015 Jun;35(6):1306-16. doi: 10.1161/ATVBAHA.114.304650. Epub 2015 Apr 2. Arterioscler Thromb Vasc Biol. 2015. PMID: 25838429 Free PMC article. Review.
Cited by
-
Role of Macrophages in Sickle Cell Disease Erythrophagocytosis and Erythropoiesis.Int J Mol Sci. 2023 Mar 28;24(7):6333. doi: 10.3390/ijms24076333. Int J Mol Sci. 2023. PMID: 37047304 Free PMC article. Review.
-
Inflammatory Dendritic Cells Contribute to Regulate the Immune Response in Sickle Cell Disease.Front Immunol. 2021 Feb 4;11:617962. doi: 10.3389/fimmu.2020.617962. eCollection 2020. Front Immunol. 2021. PMID: 33613546 Free PMC article.
References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

