Chimeric Hemagglutinin-Based Influenza Virus Vaccines Induce Protective Stalk-Specific Humoral Immunity and Cellular Responses in Mice
- PMID: 31032479
- PMCID: PMC6485968
- DOI: 10.4049/immunohorizons.1900022
Chimeric Hemagglutinin-Based Influenza Virus Vaccines Induce Protective Stalk-Specific Humoral Immunity and Cellular Responses in Mice
Abstract
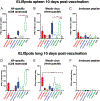
The high variation of the influenza virus hemagglutinin (HA), particularly of its immunodominant head epitopes, makes it necessary to reformulate seasonal influenza virus vaccines every year. Novel influenza virus vaccines that redirect the immune response toward conserved epitopes of the HA stalk domain should afford broad and durable protection. Sequential immunization with chimeric HAs (cHAs) that express the same conserved HA stalk and distinct exotic HA heads has been shown to elicit high levels of broadly cross-reactive Abs. In the current mouse immunization studies, we tested this strategy using inactivated split virion cHA influenza virus vaccines (IIV) without adjuvant or adjuvanted with AS01 or AS03 to measure the impact of adjuvant on the Ab response. The vaccines elicited high levels of cross-reactive Abs that showed activity in an Ab-dependent, cell-mediated cytotoxicity reporter assay and were protective in a mouse viral challenge model after serum transfer. In addition, T cell responses to adjuvanted IIV were compared with responses to a cHA-expressing live attenuated influenza virus vaccine (LAIV). A strong but transient induction of Ag-specific T cells was observed in the spleens of mice vaccinated with LAIV. Interestingly, IIV also induced T cells, which were successfully recalled upon viral challenge. Groups that received AS01-adjuvanted IIV or LAIV 4 wk before the challenge showed the lowest level of viral replication (i.e., the highest level of protection). These studies provide evidence that broadly cross-reactive Abs elicited by cHA vaccination demonstrate Fc-mediated activity. In addition, cHA vaccination induced Ag-specific cellular responses that can contribute to protection upon infection.
Figures



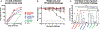

Similar articles
-
Sequential Immunization With Live-Attenuated Chimeric Hemagglutinin-Based Vaccines Confers Heterosubtypic Immunity Against Influenza A Viruses in a Preclinical Ferret Model.Front Immunol. 2019 Apr 10;10:756. doi: 10.3389/fimmu.2019.00756. eCollection 2019. Front Immunol. 2019. PMID: 31105689 Free PMC article.
-
A chimeric haemagglutinin-based universal influenza virus vaccine boosts human cellular immune responses directed towards the conserved haemagglutinin stalk domain and the viral nucleoprotein.EBioMedicine. 2024 Jun;104:105153. doi: 10.1016/j.ebiom.2024.105153. Epub 2024 May 27. EBioMedicine. 2024. PMID: 38805853 Free PMC article. Clinical Trial.
-
Vaccination with Vesicular Stomatitis Virus-Vectored Chimeric Hemagglutinins Protects Mice against Divergent Influenza Virus Challenge Strains.J Virol. 2015 Dec 16;90(5):2544-50. doi: 10.1128/JVI.02598-15. J Virol. 2015. PMID: 26676789 Free PMC article.
-
Next-Generation Influenza HA Immunogens and Adjuvants in Pursuit of a Broadly Protective Vaccine.Viruses. 2021 Mar 24;13(4):546. doi: 10.3390/v13040546. Viruses. 2021. PMID: 33805245 Free PMC article. Review.
-
The Quest for a Truly Universal Influenza Vaccine.Front Cell Infect Microbiol. 2019 Oct 10;9:344. doi: 10.3389/fcimb.2019.00344. eCollection 2019. Front Cell Infect Microbiol. 2019. PMID: 31649895 Free PMC article. Review.
Cited by
-
Supplementation of H7N9 Virus-Like Particle Vaccine With Recombinant Epitope Antigen Confers Full Protection Against Antigenically Divergent H7N9 Virus in Chickens.Front Immunol. 2022 Feb 21;13:785975. doi: 10.3389/fimmu.2022.785975. eCollection 2022. Front Immunol. 2022. PMID: 35265069 Free PMC article.
-
An Inactivated Influenza Virus Vaccine Approach to Targeting the Conserved Hemagglutinin Stalk and M2e Domains.Vaccines (Basel). 2019 Sep 18;7(3):117. doi: 10.3390/vaccines7030117. Vaccines (Basel). 2019. PMID: 31540436 Free PMC article.
-
Activity of human serum antibodies in an influenza virus hemagglutinin stalk-based ADCC reporter assay correlates with activity in a CD107a degranulation assay.Vaccine. 2020 Feb 18;38(8):1953-1961. doi: 10.1016/j.vaccine.2020.01.008. Epub 2020 Jan 17. Vaccine. 2020. PMID: 31959425 Free PMC article.
-
Administration of antigenically distinct influenza viral particle combinations as an influenza vaccine strategy.PLoS Pathog. 2025 Jan 22;21(1):e1012878. doi: 10.1371/journal.ppat.1012878. eCollection 2025 Jan. PLoS Pathog. 2025. PMID: 39841684 Free PMC article.
-
Headless hemagglutinin-containing influenza viral particles direct immune responses toward more conserved epitopes.J Virol. 2024 Oct 22;98(10):e0116624. doi: 10.1128/jvi.01166-24. Epub 2024 Sep 26. J Virol. 2024. PMID: 39324791 Free PMC article.
References
-
- Krammer F, and Palese P. 2015. Advances in the development of influenza virus vaccines. Nat. Rev. Drug Discov 14: 167–182. - PubMed
-
- Gerdil C 2003. The annual production cycle for influenza vaccine. Vaccine 21: 1776–1779. - PubMed
-
- Ohmit SE, Petrie JG, Cross RT, Johnson E, and Monto AS. 2011. Influenza hemagglutination-inhibition antibody titer as a correlate of vaccine-induced protection. J. Infect. Dis 204: 1879–1885. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

