Inhibitors of DAG metabolism suppress CCR2 signalling in human monocytes
- PMID: 31032885
- PMCID: PMC6609539
- DOI: 10.1111/bph.14695
Inhibitors of DAG metabolism suppress CCR2 signalling in human monocytes
Abstract
Background and purpose: CCL2 is an inflammatory chemokine that stimulates the recruitment of monocytes into tissue via activation of the GPCR CCR2.
Experimental approach: Freshly isolated human monocytes and THP-1 cells were used. Fura-2 loaded cells were used to measure intracellular Ca2+ responses. Transwell migration to measure chemotaxis. siRNA-mediated gene knock-down was used to support pharmacological approaches.
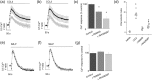
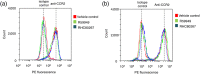
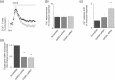
Key results: CCL2 evoked intracellular Ca2+ signals and stimulated migration in THP-1 monocytic cells and human CD14+ monocytes in a CCR2-dependent fashion. Attenuation of DAG catabolism in monocytes by inhibiting DAG kinase (R59949) or DAG lipase (RHC80267) activity suppressed CCL2-evoked Ca2+ signalling and transwell migration in monocytes. These effects were not due to a reduction in the number of cell surface CCR2. The effect of inhibiting DAG kinase or DAG lipase could be mimicked by addition of the DAG analogue 1-oleoyl-2-acetyl-sn-glycerol (OAG) but was not rescued by application of exogenous phosphatidylinositol 4,5-bisphosphate. Suppressive effects of R59949, RHC80267, and OAG were partially or fully reversed by Gö6983 (pan PKC isoenzyme inhibitor) but not by Gö6976 (PKCα and PKCβ inhibitor). RNAi-mediated knock-down of DAG kinase α isoenzyme modulated CCL2-evoked Ca2+ responses in THP-1 cells.
Conclusions and implications: Taken together, these data suggest that DAG production resulting from CCR2 activation is metabolised by both DAG kinase and DAG lipase pathways in monocytes and that pharmacological inhibition of DAG catabolism or application suppresses signalling on the CCL2-CCR2 axis via a mechanism dependent upon a PKC isoenzyme that is sensitive to Gö6983 but not Gö6976.
© 2019 The British Pharmacological Society.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures





Similar articles
-
Protein kinase C beta is required for human monocyte chemotaxis to MCP-1.J Biol Chem. 2003 Jul 11;278(28):25317-22. doi: 10.1074/jbc.M304182200. Epub 2003 Apr 30. J Biol Chem. 2003. PMID: 12724308
-
P2Y₆ receptor inhibition perturbs CCL2-evoked signalling in human monocytic and peripheral blood mononuclear cells.J Cell Sci. 2014 Nov 15;127(Pt 22):4964-73. doi: 10.1242/jcs.159012. Epub 2014 Sep 30. J Cell Sci. 2014. PMID: 25271060 Free PMC article.
-
The obesity and inflammatory marker haptoglobin attracts monocytes via interaction with chemokine (C-C motif) receptor 2 (CCR2).BMC Biol. 2009 Dec 17;7:87. doi: 10.1186/1741-7007-7-87. BMC Biol. 2009. PMID: 20017911 Free PMC article.
-
Role of the CCL2-CCR2 signalling axis in cancer: Mechanisms and therapeutic targeting.Cell Prolif. 2021 Oct;54(10):e13115. doi: 10.1111/cpr.13115. Epub 2021 Aug 31. Cell Prolif. 2021. PMID: 34464477 Free PMC article. Review.
-
Targeting the CCL2/CCR2 Axis in Cancer Immunotherapy: One Stone, Three Birds?Front Immunol. 2021 Nov 3;12:771210. doi: 10.3389/fimmu.2021.771210. eCollection 2021. Front Immunol. 2021. PMID: 34804061 Free PMC article. Review.
Cited by
-
Transcriptional and Post-Translational Regulation of Junctional Adhesion Molecule-B (JAM-B) in Leukocytes under Inflammatory Stimuli.Int J Mol Sci. 2022 Aug 3;23(15):8646. doi: 10.3390/ijms23158646. Int J Mol Sci. 2022. PMID: 35955781 Free PMC article.
-
The human amniotic fluid stem cell secretome triggers intracellular Ca2+ oscillations, NF-κB nuclear translocation and tube formation in human endothelial colony-forming cells.J Cell Mol Med. 2021 Aug;25(16):8074-8086. doi: 10.1111/jcmm.16739. Epub 2021 Jul 20. J Cell Mol Med. 2021. PMID: 34288391 Free PMC article.
-
Cell lineage-specific methylome and genome alterations in gout.Aging (Albany NY). 2021 Jan 20;13(3):3843-3865. doi: 10.18632/aging.202353. Epub 2021 Jan 20. Aging (Albany NY). 2021. PMID: 33493135 Free PMC article.
References
-
- Assani, K. , Shrestha, C. L. , Robledo‐Avila, F. , Rajaram, M. V. , Partida‐Sanchez, S. , Schlesinger, L. S. , & Kopp, B. T. (2017). Human cystic fibrosis macrophages have defective calcium‐dependent protein kinase C activation of the NADPH oxidase, an effect augmented by Burkholderia cenocepacia . Journal of Immunology, 198, 1985–1994. 10.4049/jimmunol.1502609 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

