Cas9-mediated mutagenesis of potato starch-branching enzymes generates a range of tuber starch phenotypes
- PMID: 31033104
- PMCID: PMC6835119
- DOI: 10.1111/pbi.13137
Cas9-mediated mutagenesis of potato starch-branching enzymes generates a range of tuber starch phenotypes
Abstract
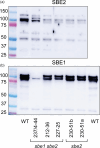
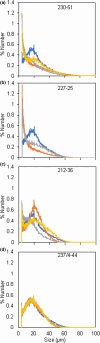
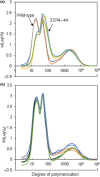
We investigated whether Cas9-mediated mutagenesis of starch-branching enzymes (SBEs) in tetraploid potatoes could generate tuber starches with a range of distinct properties. Constructs containing the Cas9 gene and sgRNAs targeting SBE1, SBE2 or both genes were introduced by Agrobacterium-mediated transformation or by PEG-mediated delivery into protoplasts. Outcomes included lines with mutations in all or only some of the homoeoalleles of SBE genes and lines in which homoeoalleles carried several different mutations. DNA delivery into protoplasts resulted in mutants with no detectable Cas9 gene, suggesting the absence of foreign DNA. Selected mutants with starch granule abnormalities had reductions in tuber SBE1 and/or SBE2 protein that were broadly in line with expectations from genotype analysis. Strong reduction in both SBE isoforms created an extreme starch phenotype, as reported previously for low-SBE potato tubers. HPLC-SEC and 1 H NMR revealed a decrease in short amylopectin chains, an increase in long chains and a large reduction in branching frequency relative to wild-type starch. Mutants with strong reductions in SBE2 protein alone had near-normal amylopectin chain-length distributions and only small reductions in branching frequency. However, starch granule initiation was enormously increased: cells contained many granules of <4 μm and granules with multiple hila. Thus, large reductions in both SBEs reduce amylopectin branching during granule growth, whereas reduction in SBE2 alone primarily affects numbers of starch granule initiations. Our results demonstrate that Cas9-mediated mutagenesis of SBE genes has the potential to generate new, potentially valuable starch properties without integration of foreign DNA into the genome.
Keywords: Cas9-mediated mutagenesis; potato tuber; starch granule; starch structure; starch-branching enzyme.
© 2019 The Authors. Plant Biotechnology Journal published by Society for Experimental Biology and The Association of Applied Biologists and John Wiley & Sons Ltd.
Conflict of interest statement
The authors declare no conflict of interests.
Figures



References
-
- Andersson, M. , Melander, M. , Pojmark, P. , Larsson, H. , Bulow, L. and Hofvander, P. (2006) Targeted gene suppression by RNA interference: an efficient method for production of high‐amylose potato lines. J. Biotechnol. 123, 137–148. - PubMed
-
- Andersson, M. , Turesson, H. , Olsson, N. , Fält, A.S. , Ohlsson, P. , Gonzalez, M.N. , Samuelsson, M. et al. (2018) Genome editing in potato via CRISPR‐Cas9 ribonucleoprotein delivery. Physiol. Plant. 164, 378–384. - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
- BBS/E/J/00000020/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/R012512/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/L014130/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BBS/E/J/000PR9799/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
- BB/J004561/1/BB_/Biotechnology and Biological Sciences Research Council/United Kingdom
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

