Paradigms of Dynamic Control of Thyroid Hormone Signaling
- PMID: 31033998
- PMCID: PMC6596318
- DOI: 10.1210/er.2018-00275
Paradigms of Dynamic Control of Thyroid Hormone Signaling
Abstract
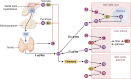
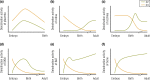
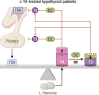
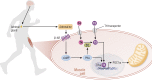
Thyroid hormone (TH) molecules enter cells via membrane transporters and, depending on the cell type, can be activated (i.e., T4 to T3 conversion) or inactivated (i.e., T3 to 3,3'-diiodo-l-thyronine or T4 to reverse T3 conversion). These reactions are catalyzed by the deiodinases. The biologically active hormone, T3, eventually binds to intracellular TH receptors (TRs), TRα and TRβ, and initiate TH signaling, that is, regulation of target genes and other metabolic pathways. At least three families of transmembrane transporters, MCT, OATP, and LAT, facilitate the entry of TH into cells, which follow the gradient of free hormone between the extracellular fluid and the cytoplasm. Inactivation or marked downregulation of TH transporters can dampen TH signaling. At the same time, dynamic modifications in the expression or activity of TRs and transcriptional coregulators can affect positively or negatively the intensity of TH signaling. However, the deiodinases are the element that provides greatest amplitude in dynamic control of TH signaling. Cells that express the activating deiodinase DIO2 can rapidly enhance TH signaling due to intracellular buildup of T3. In contrast, TH signaling is dampened in cells that express the inactivating deiodinase DIO3. This explains how THs can regulate pathways in development, metabolism, and growth, despite rather stable levels in the circulation. As a consequence, TH signaling is unique for each cell (tissue or organ), depending on circulating TH levels and on the exclusive blend of transporters, deiodinases, and TRs present in each cell. In this review we explore the key mechanisms underlying customization of TH signaling during development, in health and in disease states.
Copyright © 2019 Endocrine Society.
Figures







References
-
- Bianco AC, Salvatore D, Gereben B, Berry MJ, Larsen PR. Biochemistry, cellular and molecular biology, and physiological roles of the iodothyronine selenodeiodinases. Endocr Rev. 2002;23(1):38–89. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

