Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression
- PMID: 31034495
- PMCID: PMC6488070
- DOI: 10.1371/journal.pone.0216055
Cancer cell-induced neutrophil extracellular traps promote both hypercoagulability and cancer progression
Abstract
Introduction: Neutrophils can generate extracellular net-like structures by releasing their DNA-histone complexes and antimicrobial peptides, which is called neutrophil extracellular traps (NETs). Various stimuli can induce NET formation. In particular, neutrophils and NET formation are abundant in tumor tissue. This study investigated how cancer cells induce NET formation and whether this NET formation promotes plasma thrombin generation and cancer progression.
Methods: Induction of NET formation by a pancreatic cancer cell line (AsPC-1) was assessed by measuring the histone-DNA complex level. The endogenous thrombin potential (ETP) was measured by thrombin generation assay. In vitro migration, invasion, and tubule formation assays were performed. The circulating levels of NET markers and hypercoagulability markers were assessed in 62 patients with pancreatobiliary malignancy and 30 healthy controls.
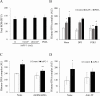
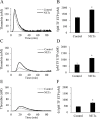
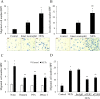
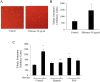
Results: AsPC-1 significantly induced NET formation in a dose-dependent manner. Conditioned medium (CM) from AsPC-1 also induced NETs. Interestingly, NET-formation was abolished by heat-inactivated CM, but not by lipid-extracted CM, suggesting an important role of protein components. A reactive oxygen species inhibitor did not inhibit cancer cell-induced NET formation, but prostaglandin E1 (PGE1, cyclic adenosine monophosphate inducer) and antithrombin did. NETs significantly increased ETP of normal plasma. Of note, NETs promoted cancer cell migration and invasion as well as angiogenesis, which were inhibited by histone-binding agents (heparin, polysialic acid), a DNA-degrading enzyme, and Toll-like receptor neutralizing antibodies. In patients with pancreatobiliary malignancy, elevated NET markers correlated well with hypercoagulability makers.
Conclusion: Our findings indicate that cancer cell-induced NET formation enhances both hypercoagulability and cancer progression and suggest that inhibitors of NET formation such as PGE1 and antithrombin can be potential therapeutics to reduce both hypercoagulability and cancer progression.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical

