The conservation and functionality of the oxygen-sensing enzyme Factor Inhibiting HIF (FIH) in non-vertebrates
- PMID: 31034531
- PMCID: PMC6488082
- DOI: 10.1371/journal.pone.0216134
The conservation and functionality of the oxygen-sensing enzyme Factor Inhibiting HIF (FIH) in non-vertebrates
Abstract
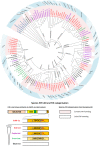
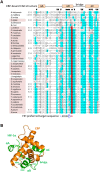
The asparaginyl hydroxylase, Factor Inhibiting HIF (FIH), is a cellular dioxygenase. Originally identified as oxygen sensor in the cellular response to hypoxia, where FIH acts as a repressor of the hypoxia inducible transcription factor alpha (HIF-α) proteins through asparaginyl hydroxylation, FIH also hydroxylates many proteins that contain ankyrin repeat domains (ARDs). Given FIH's promiscuity and the unclear functional effects of ARD hydroxylation, the biological relevance of HIF-α and ARD hydroxylation remains uncertain. Here, we have employed evolutionary and enzymatic analyses of FIH, and both HIF-α and ARD-containing substrates, in a broad range of metazoa to better understand their conservation and functional importance. Utilising Tribolium castaneum and Acropora millepora, we provide evidence that FIH from both species are able to hydroxylate HIF-α proteins, supporting conservation of this function beyond vertebrates. We further demonstrate that T. castaneum and A. millepora FIH homologs can also hydroxylate specific ARD proteins. Significantly, FIH is also conserved in several species with inefficiently-targeted or absent HIF, supporting the hypothesis of important HIF-independent functions for FIH. Overall, these data show that while oxygen-dependent HIF-α hydroxylation by FIH is highly conserved in many species, HIF-independent roles for FIH have evolved in others.
Conflict of interest statement
The authors have declared that no competing interests exist.
Figures







References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

