Site-specific replacement of phosphorothioate with alkyl phosphonate linkages enhances the therapeutic profile of gapmer ASOs by modulating interactions with cellular proteins
- PMID: 31034558
- PMCID: PMC6582325
- DOI: 10.1093/nar/gkz247
Site-specific replacement of phosphorothioate with alkyl phosphonate linkages enhances the therapeutic profile of gapmer ASOs by modulating interactions with cellular proteins
Abstract
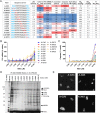
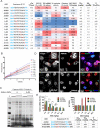
Phosphorothioate-modified antisense oligonucleotides (PS-ASOs) interact with a host of plasma, cell-surface and intracellular proteins which govern their therapeutic properties. Given the importance of PS backbone for interaction with proteins, we systematically replaced anionic PS-linkages in toxic ASOs with charge-neutral alkylphosphonate linkages. Site-specific incorporation of alkyl phosphonates altered the RNaseH1 cleavage patterns but overall rates of cleavage and activity versus the on-target gene in cells and in mice were only minimally affected. However, replacing even one PS-linkage at position 2 or 3 from the 5'-side of the DNA-gap with alkylphosphonates reduced or eliminated toxicity of several hepatotoxic gapmer ASOs. The reduction in toxicity was accompanied by the absence of nucleolar mislocalization of paraspeckle protein P54nrb, ablation of P21 mRNA elevation and caspase activation in cells, and hepatotoxicity in mice. The generality of these observations was further demonstrated for several ASOs versus multiple gene targets. Our results add to the types of structural modifications that can be used in the gap-region to enhance ASO safety and provide insights into understanding the biochemistry of PS ASO protein interactions.
© The Author(s) 2019. Published by Oxford University Press on behalf of Nucleic Acids Research.
Figures



References
-
- Crooke S.T., Wang S., Vickers T.A., Shen W., Liang X-h.. Cellular uptake and trafficking of antisense oligonucleotides. Nat. Biotech. 2017; 35:230–237. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Miscellaneous

