Mosquito Acetylcholinesterase as a Target for Novel Phenyl-Substituted Carbamates
- PMID: 31035318
- PMCID: PMC6539584
- DOI: 10.3390/ijerph16091500
Mosquito Acetylcholinesterase as a Target for Novel Phenyl-Substituted Carbamates
Abstract
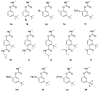
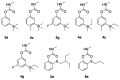
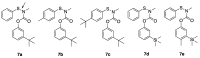
New insecticides are needed for control of disease-vectoring mosquitoes and this research evaluates the activity of new carbamate acetylcholinesterase (AChE) inhibitors. Biochemical and toxicological characterization of carbamates based on the parent structure of terbam, 3-tert-butylphenyl methylcarbamate, was performed. In vitro enzyme inhibition selectivity (Anopheles gambiae versus human) was assessed by the Ellman assay, as well as the lethality to whole insects by the World Health Organization (WHO) paper contact assay. Bromination at the phenyl C6 position increased inhibitory potency to both AChEs, whereas a 6-iodo substituent led to loss of potency, and both halogenations caused a significant reduction of mosquitocidal activity. Similarly, installation of a hexyl substituent at C6 drastically reduced inhibition of AgAChE, but showed a smaller reduction in the inhibition of hAChE. A series of 4-carboxamido analogs of the parent compound gave reduced activity against AgAChE and generally showed more activity against hAChE than AgAChE. Replacement of the 3-t-buyl group with CF3 resulted in poor anticholinesterase activity, but this compound did have measurable mosquitocidal activity. A series of methyl- and fluoro- analogs of 3-trialkylsilyl compounds were also synthesized, but unfortunately resulted in disappointing activity. Finally, a series of sulfenylated proinsecticides showed poor paper contact toxicity, but one of them had topical activity against adult female Anopheles gambiae. Overall, the analogs prepared here contributed to a better understanding of carbamate structure-activity relationships (SAR), but no new significant leads were generated.
Keywords: Anopheles gambiae; anticholinesterase; insecticide; toxicity.
Conflict of interest statement
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript, or in the decision to publish the results.
Figures
References
-
- Ware G.W., Whitacre D.M. The Pesticide Book. 6th ed. MeisterPro Information Resources; Willoughby, OH, USA: 2004. pp. 59–60.
-
- WHO . World Health Organization; [(accessed on 26 April 2019)]. WHO Recommended Insecticides for Indoor Residual Spraying against Malaria Vectors. Available online: https://www.who.int/neglected_diseases/vector_ecology/vector-control/Ins....
-
- Carlier P.R., Anderson T.D., Wong D.M., Hsu D.C., Hartsel J., Ma M., Wong E.A., Choudhury R., Lam P.C., Totrov M.M., Bloomquist J.R. Towards a species-selective acetylcholinesterase inhibitor to control the mosquito vector of malaria, Anopheles gambiae. Chem.-Biol. Interact. 2008;175:368–375. doi: 10.1016/j.cbi.2008.04.037. - DOI - PubMed
-
- Hartsel J.A., Wong D.M., Mutunga J.M., Ma M., Anderson T.D., Wysinski A., Islam R., Wong E.A., Paulson S.L., Li J., et al. Re-engineering aryl methylcarbamates to confer high selectivity for inhibition of Anopheles gambiae versus human acetylcholinesterase. Bioorg. Med. Chem. Lett. 2012;22:4593–4598. doi: 10.1016/j.bmcl.2012.05.103. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous