The IGF1 Receptor Is Involved in Follicle-Stimulating Hormone Signaling in Porcine Neonatal Sertoli Cells
- PMID: 31035547
- PMCID: PMC6571966
- DOI: 10.3390/jcm8050577
The IGF1 Receptor Is Involved in Follicle-Stimulating Hormone Signaling in Porcine Neonatal Sertoli Cells
Abstract
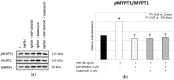
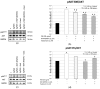
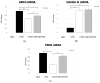
Experimental evidence has shown that the IGF1 receptor (IGF1R) is involved in testicular development during embryogenesis. More recently, data gathered from mice granulosa cells and zebrafish spermatogonia suggest that IGF1R has a role in Follicle-stimulating hormone (FSH) signaling. No evidence has been reported on this matter in Sertoli cells (SCs) so far. The aim of the study was to evaluate the role, if any, of the IGF1R in FSH signaling in SCs. The effects of FSH exposure on myosin-phosphatase 1 (MYPT1), ERK 1/2, AKT308, AKT473, c-Jun N-terminal kinase (JNK) phosphorylation and on anti-Müllerian hormone (AMH), inhibin B and FSH receptor (FSHR) mRNA levels were assessed with and without the IGF1R inhibitor NVP-AEW541 in purified and functional porcine neonatal SCs. Pre-treatment with NVP-AEW541 inhibited the FSH-induced MYPT1 and ERK 1/2 phosphorylation, decreased the FSH-dependent Protein kinase B (AKT)308 phosphorylation, but did not affect the FSH-induced AKT473 and JNK phosphorylation rate. It also interfered with the FSH-induced AMH and FSHR down-regulation. No influence was observed on the FSH-stimulated Inhibin B gene expression. Conclusion. These findings support the role of theIGF1R in FSH signaling in porcine SCs. The possible influence of IGF1 stimulation on the FSH-mediated effects on SCs should be further explored.
Keywords: Follicle-stimulating hormone; Insulin-like growth factor 1; Insulin-like growth factor 1 receptor; Sertoli cells; infertility.
Conflict of interest statement
The authors declare no conflict of interest.
Figures


References
-
- Pitetti J.L., Calvel P., Zimmermann C., Conne B., Papaioannou M.D., Aubry F., Cederroth C.R., Urner F., Fumel B., Crausaz M., et al. An essential role for insulin and IGF1 receptors in regulating sertoli cell proliferation, testis size, and FSH action in mice. Mol. Endocrinol. 2013;27:814–827. doi: 10.1210/me.2012-1258. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

