Alternative-Dose versus Standard-Dose Trivalent Influenza Vaccines for Immunocompromised Patients: A Meta-Analysis of Randomised Control Trials
- PMID: 31035712
- PMCID: PMC6571572
- DOI: 10.3390/jcm8050590
Alternative-Dose versus Standard-Dose Trivalent Influenza Vaccines for Immunocompromised Patients: A Meta-Analysis of Randomised Control Trials
Abstract
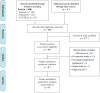
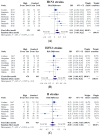
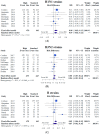
The study compared immunogenicity and safety between alternative higher-dose and standard-dose trivalent vaccines in immunocompromised individuals. A literature search was performed using the PubMed, Embase, and Cochrane databases from inception until March 2019 to identify studies comparing the immunogenicity of alternative higher-dose (including high-dose, double-dose, and booster-dose vaccines) and standard-dose trivalent influenza vaccines in patients who underwent transplantation or chemotherapy. Effect estimates from the individual studies were derived and calculated using the DerSimonian and Laird random-effect model. The protocol for this systematic review is registered with PROSPERO (number CRD42019129220). Eight relevant studies involving 1020 patients were included in the systematic review and meta-analysis. The meta-analysis demonstrated that the higher-dose strategy provided had significantly superior seroconversion and seroprotection for A/H1N1 strains than the standard dose. Regarding H3N2 and B strains, no differences in immunogenicity responses were noted. No differences in safety were observed between the vaccination strategies. Alternative higher-dose vaccination strategies appear to associate with superior immunogenicity responses for A/H1N1 strains, and the strategies were generally well tolerated in immunocompromised populations. Future studies should clarify the optimal timing, frequency and dose of vaccination and assess whether these strategies improve vaccine immunogenicity and clinical outcomes.
Keywords: booster dose; cancer; chemotherapy; double dose; high dose; immunocompromised; influenza vaccine; meta-analysis; transplant; trivalent.
Conflict of interest statement
The authors declare no conflict of interest.
Figures
References
-
- WHO Influenza (Seasonal) [(accessed on 6 November 2018)]; Available online: https://www.who.int/news-room/fact-sheets/detail/influenza-(seasonal)
-
- Kumar D., Michaels M.G., Morris M.I., Green M., Avery R.K., Liu C., Danziger-Isakov L., Stosor V., Estabrook M., Gantt S., et al. Outcomes from pandemic influenza A H1N1 infection in recipients of solid-organ transplants: A multicentre cohort study. Lancet Infect. Dis. 2010;10:521–526. doi: 10.1016/S1473-3099(10)70133-X. - DOI - PMC - PubMed
-
- Grohskopf L.A., Sokolow L.Z., Broder K.R., Walter E.B., Bresee J.S., Fry A.M., Jernigan D.B. Prevention and Control of Seasonal Influenza with Vaccines: Recommendations of the Advisory Committee on Immunization Practices—United States, 2017–2018 Influenza Season. MMWR Morb. Mortal. Wkly. Rep. 2017;66:1–20. - PMC - PubMed
LinkOut - more resources
Full Text Sources

