Transplantation and diabetes (Transdiab): a pilot randomised controlled trial of metformin in impaired glucose tolerance after kidney transplantation
- PMID: 31035960
- PMCID: PMC6489311
- DOI: 10.1186/s12882-019-1321-2
Transplantation and diabetes (Transdiab): a pilot randomised controlled trial of metformin in impaired glucose tolerance after kidney transplantation
Abstract
Background: Post transplantation diabetes mellitus (PTDM) is a common and serious complication after renal transplantation with significant morbidity and mortality. Metformin has proven benefits in the general population and might be advantageous in the prevention and management of PTDM.
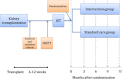
Methods: Transplantation and Diabetes (Transdiab) is a single-centre, unblinded, pilot randomised controlled trial assessing the feasibility, tolerability and efficacy of metformin after renal transplantation in patients with impaired glucose tolerance (IGT). Participants had an oral glucose tolerance test (OGTT) in the 4-12 weeks post-transplantation; those with IGT were randomised to standard care or standard care and metformin 500 mg twice daily and followed up for 12 months.
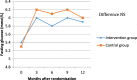
Results: Seventy eight patients had an OGTT over 24 months, 25 of them had IGT, of those, 19 patients were randomised, giving a feasibility of recruitment of 24.4%. Ten patients were randomised to metformin and 9 patients to standard care. Tolerability and efficacy was similar between the 2 groups with no serious adverse events. There was no difference in secondary outcomes relating to the metabolic profile.
Conclusions: The use of metformin post renal transplantation appeared feasible and safe. Larger randomised controlled trials (RCTs) are needed to establish and confirm the efficacy and safety of metformin post renal transplantation.
Trial registration: Australian New Zealand Clinical Trials Registry ACTRN12614001171606 . Date of registration 7/11/2014.
Keywords: Diabetic kidney disease; Kidney transplantation; Post transplant diabetes mellitus; Randomised control trial.
Conflict of interest statement
Ethics approval and consent to participate
Ethical approval has been obtained through the Northern B Health and Disability Ethics Committee of the Ministry of Health in New Zealand. Ethics approval number is 14/ STH/129. Written informed consent has been obtained from all individuals participating in the study.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures


References
-
- ANZDATA Registry. The 39th annual ANZDATA report. Australia and New Zealand Dialysis and transplant Registry, Adelaide, Australia. 2017. Available at: http://www.anzdata.org.au/v1/report_2016.html. Accessed 13 Apr 2019.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical

