Cognitively-Based Compassion Training versus cancer health education to improve health-related quality of life in survivors of solid tumor cancers and their informal caregivers: study protocol for a randomized controlled pilot trial
- PMID: 31036091
- PMCID: PMC6489281
- DOI: 10.1186/s13063-019-3320-9
Cognitively-Based Compassion Training versus cancer health education to improve health-related quality of life in survivors of solid tumor cancers and their informal caregivers: study protocol for a randomized controlled pilot trial
Abstract
Background: Cancer survivors and their informal caregivers (family members, close friends) often experience significant impairments in health-related quality of life (HRQOL), including disruptions in psychological, physical, social, and spiritual well-being both during and after primary cancer treatment. The purpose of this in-progress pilot trial is to determine acceptability and preliminary efficacy (as reflected by effect sizes) of CBCT® (Cognitively-Based Compassion Training) compared with a cancer health education (CHE) attention control to improve the primary outcome of depressive symptoms and secondary outcomes of other HRQOL domains (e.g., anxiety, fatigue), biomarkers of inflammation and diurnal cortisol rhythm, and healthcare utilization-related outcomes in both cancer survivors and informal caregivers.
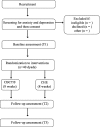
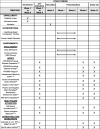
Methods: Forty dyads consisting of solid tumor survivors who have completed primary treatments (chemotherapy, radiation, surgery) and their informal caregivers, with at least one dyad member with ≥ mild depressive symptoms or anxiety, will be recruited from Tucson, Arizona, USA. Survivor-caregiver dyads will be randomized together to complete either CBCT or CHE. CBCT is a manualized, 8-week, group meditation-based intervention that starts with attention and mindfulness and builds to contemplative practices aimed at cultivating compassion to the self and others. The goal of CBCT is to challenge unexamined assumptions about feelings and behaviors, with a focus on generating spontaneous self-compassion and increased empathic responsiveness and compassion for others. CHE is an 8-week, manualized group intervention that provides cancer-specific education on various topics (e.g., cancer advocacy, survivorship wellness). Patient-reported HRQOL outcomes will be assessed before, immediately after (week 9), and 1 month after CBCT or CHE (week 13). At the same time points, stress-related biomarkers of inflammation (e.g., plasma interleukin-6) and saliva cortisol relevant for survivor and informal caregiver wellness and healthcare utilization will be measured.
Discussion: If CBCT shows acceptability, a larger trial will be warranted and appropriately powered to formally test the efficacy of this dyadic intervention. Interventions such as CBCT directed toward both survivors and caregivers may eventually fill a gap in supportive oncology care programs to improve HRQOL and healthcare utilization in both dyad members.
Trial registration: Clinicaltrials.gov, NCT03459781 . Prospectively registered on 9 March 2018.
Keywords: Active control; Cancer survivorship; Compassion meditation; Cortisol; Dyadic interdependence; Health-related quality of life; Inflammation.
Conflict of interest statement
Ethics approval and consent to participate
This study was reviewed and approved by the Institutional Review Board responsible for human subject research at The University of Arizona.
Consent for publication
Not applicable.
Competing interests
The authors declare that they have no competing interests.
Publisher’s Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Figures
References
-
- NCI. SEER cancer statistics review (CSR) 1975–2014. 2017; https://seer.cancer.gov/csr/1975_2014/. Accessed 1 May 2018, 2015.
-
- Krebber AM, Buffart LM, Kleijn G, Riepma IC, de Bree R, Leemans CR, Becker A, Brug J, van Straten A, Cuijpers P, Verdonck-de Leeuw IM. Prevalence of depression in cancer patients: a meta-analysis of diagnostic interviews and self-report instruments. Psychooncology. 2014;23(2):121–130. doi: 10.1002/pon.3409. - DOI - PMC - PubMed
Publication types
MeSH terms
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

