Ectosymbiotic bacteria at the origin of magnetoreception in a marine protist
- PMID: 31036911
- PMCID: PMC6697534
- DOI: 10.1038/s41564-019-0432-7
Ectosymbiotic bacteria at the origin of magnetoreception in a marine protist
Abstract
Mutualistic symbioses are often a source of evolutionary innovation and drivers of biological diversification1. Widely distributed in the microbial world, particularly in anoxic settings2,3, they often rely on metabolic exchanges and syntrophy2,4. Here, we report a mutualistic symbiosis observed in marine anoxic sediments between excavate protists (Symbiontida, Euglenozoa)5 and ectosymbiotic Deltaproteobacteria biomineralizing ferrimagnetic nanoparticles. Light and electron microscopy observations as well as genomic data support a multi-layered mutualism based on collective magnetotactic motility with division of labour and interspecies hydrogen-transfer-based syntrophy6. The guided motility of the consortia along the geomagnetic field is allowed by the magnetic moment of the non-motile ectosymbiotic bacteria combined with the protist motor activity, which is a unique example of eukaryotic magnetoreception7 acquired by symbiosis. The nearly complete deltaproteobacterial genome assembled from a single consortium contains a full magnetosome gene set8, but shows signs of reduction, with the probable loss of flagellar genes. Based on the metabolic gene content, the ectosymbiotic bacteria are anaerobic sulfate-reducing chemolithoautotrophs that likely reduce sulfate with hydrogen produced by hydrogenosome-like organelles6 underlying the plasma membrane of the protist. In addition to being necessary hydrogen sinks, ectosymbionts may provide organics to the protist by diffusion and predation, as shown by magnetosome-containing digestive vacuoles. Phylogenetic analyses of 16S and 18S ribosomal RNA genes from magnetotactic consortia in marine sediments across the Northern and Southern hemispheres indicate a host-ectosymbiont specificity and co-evolution. This suggests a historical acquisition of magnetoreception by a euglenozoan ancestor from Deltaproteobacteria followed by subsequent diversification. It also supports the cosmopolitan nature of this type of symbiosis in marine anoxic sediments.
Conflict of interest statement
The authors declare no competing interests.
Figures

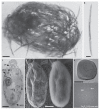

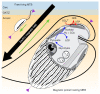
Comment in
-
Symbiotic magnetic motility.Nat Microbiol. 2019 Jul;4(7):1066-1067. doi: 10.1038/s41564-019-0489-3. Nat Microbiol. 2019. PMID: 31222171 No abstract available.
References
-
- Margulis L, Fester R. Symbiosis as a Source of Evolutionary Innovation: Speciation and Morphogenesis. MIT Press; 1991. - PubMed
-
- Bernhard JM, Buck KR, Farmer MA, Bowser SS. The Santa Barbara Basin is a symbiosis oasis. Nature. 2000;403:77–80. - PubMed
-
- Morris BEL, Henneberger R, Huber H, Moissl-Eichinger C. Microbial syntrophy: interaction for the common good. FEMS Microbiol Rev. 2013;37:384–406. - PubMed
-
- Yubuki N, Leander BS. Diversity and evolutionary history of the symbiontida (Euglenozoa) Front Ecol Evol. 2018;6:100.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

