Memory formation in the absence of experience
- PMID: 31036944
- PMCID: PMC7592289
- DOI: 10.1038/s41593-019-0389-0
Memory formation in the absence of experience
Abstract
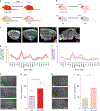
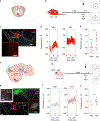
Memory is coded by patterns of neural activity in distinct circuits. Therefore, it should be possible to reverse engineer a memory by artificially creating these patterns of activity in the absence of a sensory experience. In olfactory conditioning, an odor conditioned stimulus (CS) is paired with an unconditioned stimulus (US; for example, a footshock), and the resulting CS-US association guides future behavior. Here we replaced the odor CS with optogenetic stimulation of a specific olfactory glomerulus and the US with optogenetic stimulation of distinct inputs into the ventral tegmental area that mediate either aversion or reward. In doing so, we created a fully artificial memory in mice. Similarly to a natural memory, this artificial memory depended on CS-US contingency during training, and the conditioned response was specific to the CS and reflected the US valence. Moreover, both real and implanted memories engaged overlapping brain circuits and depended on basolateral amygdala activity for expression.
Figures


Comment in
-
Memories light the corners of my mind.Nat Neurosci. 2019 Jun;22(6):845-846. doi: 10.1038/s41593-019-0390-7. Nat Neurosci. 2019. PMID: 31036943 No abstract available.
References
-
- Josselyn SA, Kohler S & Frankland PW Finding the engram. Nat. Rev. Neurosci. 16, 521–534 (2015). - PubMed
-
- Tonegawa S, Liu X, Ramirez S & Redondo R Memory engram cells have come of age. Neuron 87, 918–931 (2015). - PubMed
-
- Martin SJ & Morris RG New life in an old idea: the synaptic plasticity and memory hypothesis revisited. Hippocampus 12, 609–636 (2002). - PubMed
-
- Buck L & Axel R A novel multigene family may encode odorant receptors: a molecular basis for odor recognition. Cell 65, 175–187 (1991). - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Molecular Biology Databases

