Metabolism of remimazolam in primary human hepatocytes during continuous long-term infusion in a 3-D bioreactor system
- PMID: 31037028
- PMCID: PMC6450186
- DOI: 10.2147/DDDT.S186759
Metabolism of remimazolam in primary human hepatocytes during continuous long-term infusion in a 3-D bioreactor system
Abstract
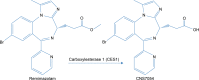
Background: Remimazolam is an ultra-short acting benzodiazepine under development for procedural sedation and general anesthesia. It is hydrolyzed by CES1 to an inactive metabolite (CNS7054).
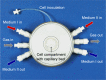
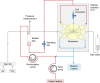
Purpose: In this study, the effect of continuous remimazolam exposure on its metabolism and on CES1 expression was investigated in a dynamic 3-D bioreactor culture model inoculated with primary human hepatocytes.
Methods: Remimazolam was continuously infused into bioreactors for 5 days at a final concentration of 3,000 ng/ml (6.8 µM). In parallel, 2-D cultures were run with cells from the same donors, but with discontinuous exposure to remimazolam.
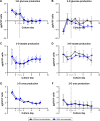
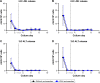
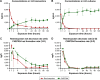
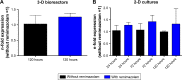
Results: Daily measurement of clinical chemistry parameters (glucose, lactate, urea, ammonia, and liver enzymes) in culture supernatants indicated no noxious effect of remimazolam on hepatocyte integrity as compared to untreated controls. Concentrations of remimazolam reached steady-state values of around 250 ng/ml within 8 hours in 3-D bioreactors whereas in 2-D cultures remimazolam concentrations declined to almost zero within the same time frame. Levels of CNS7054 showed an inverse time-course reaching average values of 1,350 ng/ml in perfused 3-D bioreactors resp. 2,800 ng/ml in static 2-D cultures. Analysis of mRNA expression levels of CES1 indicated no changes in gene expression over the culture period.
Conclusion: The results indicated a stable metabolism of remimazolam during 5 days of continuous exposure to clinically relevant concentrations of the drug. Moreover, there was no evidence for a harmful effect of remimazolam exposure on the integrity and metabolic activity of in vitro cultivated primary human hepatocytes.
Keywords: CNS7054; benzodiazepine; carboxylesterase 1; metabolite; steady state.
Figures





References
-
- Kilpatrick GJ, McIntyre MS, Cox RF, et al. CNS 7056: a novel ultra-short-acting benzodiazepine. Anesthesiology. 2007;107(1):60–66. - PubMed
-
- Antonik LJ, Goldwater DR, Kilpatrick GJ, Tilbrook GS, Borkett KM. A placebo- and midazolam-controlled phase I single ascending-dose study evaluating the safety, pharmacokinetics, and pharmacodynamics of remimazolam (CNS 7056): Part I. safety, efficacy, and basic pharmacokinetics. Anesth Analg. 2012;115(2):274–283. - PubMed
-
- Borkett KM, Riff DS, Schwartz HI, et al. A phase IIA, randomized, double-blind study of remimazolam (CNS 7056) versus midazolam for sedation in upper gastrointestinal endoscopy. Anesth Analg. 2015;120(4):771–780. - PubMed
-
- Pambianco DJ, Borkett KM, Riff DS, et al. A phase IIb study comparing the safety and efficacy of remimazolam and midazolam in patients undergoing colonoscopy. Gastrointest Endosc. 2016;83(5):984–992. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

