Berberine Protects Glomerular Podocytes via Inhibiting Drp1-Mediated Mitochondrial Fission and Dysfunction
- PMID: 31037132
- PMCID: PMC6485199
- DOI: 10.7150/thno.30640
Berberine Protects Glomerular Podocytes via Inhibiting Drp1-Mediated Mitochondrial Fission and Dysfunction
Abstract
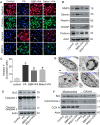
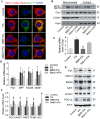
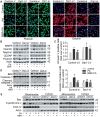
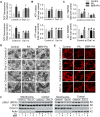
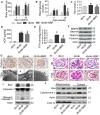
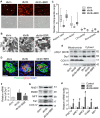
Elevated levels of plasma free fatty acid (FFA) and disturbed mitochondrial dynamics play crucial roles in the pathogenesis of diabetic kidney disease (DKD). However, the mechanisms by which FFA leads to mitochondrial damage in glomerular podocytes of DKD and the effects of Berberine (BBR) on podocytes are not fully understood. Methods: Using the db/db diabetic mice model and cultured mouse podocytes, we investigated the molecular mechanism of FFA-induced disturbance of mitochondrial dynamics in podocytes and testified the effects of BBR on regulating mitochondrial dysfunction, podocyte apoptosis and glomerulopathy in the progression of DKD. Results: Intragastric administration of BBR for 8 weeks in db/db mice significantly reversed glucose and lipid metabolism disorders, podocyte damage, basement membrane thickening, mesangial expansion and glomerulosclerosis. BBR strongly inhibited podocyte apoptosis, increased reactive oxygen species (ROS) generation, mitochondrial fragmentation and dysfunction both in vivo and in vitro. Mechanistically, BBR could stabilize mitochondrial morphology in podocytes via abolishing palmitic acid (PA)-induced activation of dynamin-related protein 1 (Drp1). Conclusions: Our study demonstrated for the first time that BBR may have a previously unrecognized role in protecting glomerulus and podocytes via positively regulating Drp1-mediated mitochondrial dynamics. It might serve as a novel therapeutic drug for the treatment of DKD.
Keywords: Berberine; diabetic kidney disease; dynamin-related protein 1; mitochondrial fission; podocyte.
Conflict of interest statement
Competing Interests: The authors have declared that no competing interest exists.
Figures


References
-
- Chan DC. Fusion and fission: interlinked processes critical for mitochondrial health. Annu Rev Genet. 2012;46:265–87. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

