Rescue of premature aging defects in Cockayne syndrome stem cells by CRISPR/Cas9-mediated gene correction
- PMID: 31037510
- PMCID: PMC6949206
- DOI: 10.1007/s13238-019-0623-2
Rescue of premature aging defects in Cockayne syndrome stem cells by CRISPR/Cas9-mediated gene correction
Erratum in
-
Correction to: Rescue of premature aging defects in Cockayne syndrome stem cells by CRISPR/Cas9-mediated gene correction.Protein Cell. 2022 Aug;13(8):623-625. doi: 10.1007/s13238-021-00901-3. Protein Cell. 2022. PMID: 35032293 Free PMC article. No abstract available.
Abstract
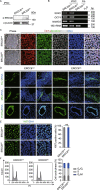
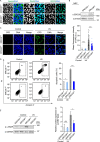
Cockayne syndrome (CS) is a rare autosomal recessive inherited disorder characterized by a variety of clinical features, including increased sensitivity to sunlight, progressive neurological abnormalities, and the appearance of premature aging. However, the pathogenesis of CS remains unclear due to the limitations of current disease models. Here, we generate integration-free induced pluripotent stem cells (iPSCs) from fibroblasts from a CS patient bearing mutations in CSB/ERCC6 gene and further derive isogenic gene-corrected CS-iPSCs (GC-iPSCs) using the CRISPR/Cas9 system. CS-associated phenotypic defects are recapitulated in CS-iPSC-derived mesenchymal stem cells (MSCs) and neural stem cells (NSCs), both of which display increased susceptibility to DNA damage stress. Premature aging defects in CS-MSCs are rescued by the targeted correction of mutant ERCC6. We next map the transcriptomic landscapes in CS-iPSCs and GC-iPSCs and their somatic stem cell derivatives (MSCs and NSCs) in the absence or presence of ultraviolet (UV) and replicative stresses, revealing that defects in DNA repair account for CS pathologies. Moreover, we generate autologous GC-MSCs free of pathogenic mutation under a cGMP (Current Good Manufacturing Practice)-compliant condition, which hold potential for use as improved biomaterials for future stem cell replacement therapy for CS. Collectively, our models demonstrate novel disease features and molecular mechanisms and lay a foundation for the development of novel therapeutic strategies to treat CS.
Keywords: CRISPR/Cas9; Cockayne syndrome; disease modelling; gene correction; mesenchymal stem cell; neural stem cell.
Figures






Similar articles
-
Perspectives in the investigation of Cockayne syndrome group B neurological disease: the utility of patient-derived brain organoid models.J Zhejiang Univ Sci B. 2024 Oct 2;25(10):878-889. doi: 10.1631/jzus.B2300712. J Zhejiang Univ Sci B. 2024. PMID: 39420523 Free PMC article. Review.
-
Cockayne syndrome proteins CSA and CSB maintain mitochondrial homeostasis through NAD+ signaling.Aging Cell. 2020 Dec;19(12):e13268. doi: 10.1111/acel.13268. Epub 2020 Nov 9. Aging Cell. 2020. PMID: 33166073 Free PMC article.
-
Evidence for premature aging due to oxidative stress in iPSCs from Cockayne syndrome.Hum Mol Genet. 2012 Sep 1;21(17):3825-34. doi: 10.1093/hmg/dds211. Epub 2012 Jun 1. Hum Mol Genet. 2012. PMID: 22661500 Free PMC article.
-
Identification and Characterization of a Novel Recurrent ERCC6 Variant in Patients with a Severe Form of Cockayne Syndrome B.Genes (Basel). 2021 Nov 29;12(12):1922. doi: 10.3390/genes12121922. Genes (Basel). 2021. PMID: 34946871 Free PMC article.
-
Cockayne Syndrome Group B (CSB): The Regulatory Framework Governing the Multifunctional Protein and Its Plausible Role in Cancer.Cells. 2021 Apr 10;10(4):866. doi: 10.3390/cells10040866. Cells. 2021. PMID: 33920220 Free PMC article. Review.
Cited by
-
Recent Advances in CRISPR/Cas9 Delivery Approaches for Therapeutic Gene Editing of Stem Cells.Stem Cell Rev Rep. 2023 Nov;19(8):2576-2596. doi: 10.1007/s12015-023-10585-3. Epub 2023 Sep 18. Stem Cell Rev Rep. 2023. PMID: 37723364 Free PMC article. Review.
-
Perspectives in the investigation of Cockayne syndrome group B neurological disease: the utility of patient-derived brain organoid models.J Zhejiang Univ Sci B. 2024 Oct 2;25(10):878-889. doi: 10.1631/jzus.B2300712. J Zhejiang Univ Sci B. 2024. PMID: 39420523 Free PMC article. Review.
-
Cross-species metabolomic analysis identifies uridine as a potent regeneration promoting factor.Cell Discov. 2022 Feb 1;8(1):6. doi: 10.1038/s41421-021-00361-3. Cell Discov. 2022. PMID: 35102134 Free PMC article.
-
The ageing epigenome and its rejuvenation.Nat Rev Mol Cell Biol. 2020 Mar;21(3):137-150. doi: 10.1038/s41580-019-0204-5. Epub 2020 Feb 4. Nat Rev Mol Cell Biol. 2020. PMID: 32020082 Review.
-
Cellular senescence, neuroinflammation, and microRNAs: Possible interactions driving aging and neurodegeneration in the hippocampal neurogenic niche.Aging Brain. 2025 Jun 12;8:100141. doi: 10.1016/j.nbas.2025.100141. eCollection 2025. Aging Brain. 2025. PMID: 40584008 Free PMC article. Review.
References
-
- Andressoo JO, Mitchell JR, de Wit J, Hoogstraten D, Volker M, Toussaint W, Speksnijder E, Beems RB, van Steeg H, Jans J, et al. An Xpd mouse model for the combined xeroderma pigmentosum/Cockayne syndrome exhibiting both cancer predisposition and segmental progeria. Cancer Cell. 2006;10:121–132. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

