Aberrant splicing contributes to severe α-spectrin-linked congenital hemolytic anemia
- PMID: 31038472
- PMCID: PMC6597203
- DOI: 10.1172/JCI127195
Aberrant splicing contributes to severe α-spectrin-linked congenital hemolytic anemia
Abstract
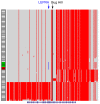
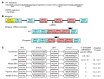
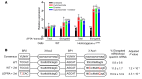
The etiology of severe hemolytic anemia in most patients with recessive hereditary spherocytosis (rHS) and the related disorder hereditary pyropoikilocytosis (HPP) is unknown. Whole exome sequencing of DNA from probands of 24 rHS or HPP kindreds identified numerous mutations in erythrocyte membrane α-spectrin (SPTA1). Twenty-eight mutations were novel, with null alleles frequently found in trans to missense mutations. No mutations were identified in a third of SPTA1 alleles (17/48). Whole genome sequencing revealed linkage disequilibrium between the common rHS-linked α-spectrinBug Hill polymorphism and a rare intron 30 variant in all 17 mutation-negative alleles. In vitro minigene studies and in vivo splicing analyses revealed the intron 30 variant changes a weak alternate branch point (BP) to a strong BP. This change leads to increased utilization of an alternate 3' splice acceptor site, perturbing normal α-spectrin mRNA splicing and creating an elongated mRNA transcript. In vivo mRNA stability studies revealed the newly created termination codon in the elongated transcript activates nonsense mediated decay leading to spectrin deficiency. These results demonstrate a unique mechanism of human genetic disease contributes to the etiology of a third of cases of rHS, facilitating diagnosis and treatment of severe anemia, and identifying a new target for therapeutic manipulation.
Keywords: Genetic diseases; Genetics; Hematology.
Conflict of interest statement
Figures


Comment in
-
Anemia lurking in introns.J Clin Invest. 2019 Jun 4;129(7):2655-2657. doi: 10.1172/JCI129443. eCollection 2019 Jun 4. J Clin Invest. 2019. PMID: 31162137 Free PMC article.

