Sensitive and adaptable pharmacological control of CAR T cells through extracellular receptor dimerization
- PMID: 31039141
- PMCID: PMC6629089
- DOI: 10.1172/jci.insight.124430
Sensitive and adaptable pharmacological control of CAR T cells through extracellular receptor dimerization
Abstract
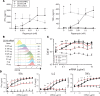
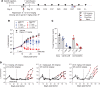
Chimeric antigen receptor (CAR) T cell therapies have achieved promising outcomes in several cancers, however more challenging oncology indications may necessitate advanced antigen receptor designs and functions. Here we describe a bipartite receptor system comprised of separate antigen targeting and signal transduction polypeptides, each containing an extracellular dimerization domain. We demonstrate that T cell activation remains antigen dependent but can only be achieved in the presence of a dimerizing drug, rapamycin. Studies performed in vitro and in xenograft mouse models illustrate equivalent to superior anti-tumor potency compared to currently used CAR designs, and at rapamycin concentrations well below immunosuppressive levels. We further show that the extracellular positioning of the dimerization domains enables the administration of recombinant re-targeting modules, potentially extending antigen targeting. Overall, this novel regulatable CAR design has exquisite drug sensitivity, provides robust anti-tumor responses, and is uniquely flexible for multiplex antigen targeting or retargeting, which may further assist the development of safe, potent and durable T cell therapeutics.
Keywords: Cancer immunotherapy; Gene therapy; Leukemias; Oncology; Therapeutics.
Conflict of interest statement
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

