Structural changes in the transport cycle of the mitochondrial ADP/ATP carrier
- PMID: 31039524
- PMCID: PMC6700394
- DOI: 10.1016/j.sbi.2019.03.029
Structural changes in the transport cycle of the mitochondrial ADP/ATP carrier
Abstract
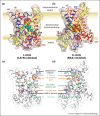
The mitochondrial ADP/ATP carrier, also called adenine nucleotide translocase, accomplishes one of the most important transport activities in eukaryotic cells, importing ADP into the mitochondrial matrix for ATP synthesis, and exporting ATP to fuel cellular activities. In the transport cycle, the carrier changes between a cytoplasmic and matrix state, in which the central substrate binding site is alternately accessible to these compartments. A structure of a cytoplasmic state was known, but recently, a structure of a matrix-state in complex with bongkrekic acid was solved. Comparison of the two states explains the function of highly conserved sequence features and reveals that the transport mechanism is unique, involving the coordinated movement of six dynamic elements around a central translocation pathway.
Copyright © 2019 The Authors. Published by Elsevier Ltd.. All rights reserved.
Figures




References
-
- Kunji E.R.S., Aleksandrova A., King M.S., Majd H., Ashton V.L., Cerson E., Springett R., Kibalchenko M., Tavoulari S., Crichton P.G. The transport mechanism of the mitochondrial ADP/ATP carrier. Biochim Biophys Acta. 2016;1863:2379–2393. - PubMed
-
Comprehensive review of the structure and function of mitochondrial ADP/ATP carriers, referencing earlier studies.
-
- Garrett R., Grisham C.M. edn 4. Brooks/Cole; Cengage Learning; International ed. Australia; United Kingdom: 2010. Biochemistry.
-
- Buono M.J., Kolkhorst F.W. Estimating ATP resynthesis during a marathon run: a method to introduce metabolism. Physiol Educ. 2001;25:70–71.
-
- Kunji E.R.S. The mitochondrial family of transport proteins. In: Egelman E., editor. vol 8. Elsevier; 2012. pp. 174–205. (Comprehensive Biophysics).
-
- Monne M., Palmieri F. Antiporters of the mitochondrial carrier family. Curr Top Membr. 2014;73:289–320. - PubMed
-
Review covering other members of the mitochondrial carrier family.
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

