Human interleukin-2 receptor β mutations associated with defects in immunity and peripheral tolerance
- PMID: 31040185
- PMCID: PMC6547869
- DOI: 10.1084/jem.20182304
Human interleukin-2 receptor β mutations associated with defects in immunity and peripheral tolerance
Abstract
Interleukin-2, which conveys essential signals for immunity, operates through a heterotrimeric receptor. Here we identify human interleukin-2 receptor (IL-2R) β chain (IL2RB) gene defects as a cause of life-threatening immune dysregulation. We report three homozygous mutations in the IL2RB gene of eight individuals from four consanguineous families that cause disease by distinct mechanisms. Nearly all patients presented with autoantibodies, hypergammaglobulinemia, bowel inflammation, dermatological abnormalities, lymphadenopathy, and cytomegalovirus disease. Patient T lymphocytes lacked surface expression of IL-2Rβ and were unable to respond to IL-2 stimulation. By contrast, natural killer cells retained partial IL-2Rβ expression and function. IL-2Rβ loss of function was recapitulated in a recombinant system in which IL2RB mutations caused reduced surface expression and IL-2 binding. Stem cell transplant ameliorated clinical symptoms in one patient; forced expression of wild-type IL-2Rβ also increased the IL-2 responsiveness of patient T lymphocytes in vitro. Insights from these patients can inform the development of IL-2-based therapeutics for immunological diseases and cancer.
This is a work of the U.S. Government and is not subject to copyright protection in the United States. Foreign copyrights may apply.
Figures

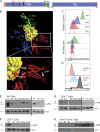
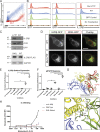

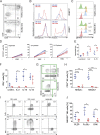
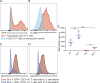
Comment in
-
IL2RB maintains immune harmony.J Exp Med. 2019 Jun 3;216(6):1231-1233. doi: 10.1084/jem.20190546. Epub 2019 May 8. J Exp Med. 2019. PMID: 31068380 Free PMC article.
References
-
- Abraham M.J., Murtola T., Schulz R., Páll S., Smith J.C., Hess B., and Lindahl E.. 2015. GROMACS: High performance molecular simulations through multi-level parallelism from laptops to supercomputers. SoftwareX. 1-2:19–25. 10.1016/j.softx.2015.06.001 - DOI
-
- Bielekova B., Catalfamo M., Reichert-Scrivner S., Packer A., Cerna M., Waldmann T.A., McFarland H., Henkart P.A., and Martin R.. 2006. Regulatory CD56(bright) natural killer cells mediate immunomodulatory effects of IL-2Ralpha-targeted therapy (daclizumab) in multiple sclerosis. Proc. Natl. Acad. Sci. USA. 103:5941–5946. 10.1073/pnas.0601335103 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
- Actions
- Actions
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
Molecular Biology Databases

