Binding of Staphylococcus aureus Protein A to von Willebrand Factor Is Regulated by Mechanical Force
- PMID: 31040240
- PMCID: PMC6495375
- DOI: 10.1128/mBio.00555-19
Binding of Staphylococcus aureus Protein A to von Willebrand Factor Is Regulated by Mechanical Force
Abstract
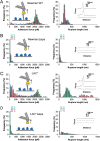
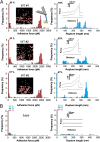
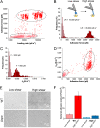
Binding of Staphylococcus aureus to the large plasma glycoprotein von Willebrand factor (vWF) is controlled by hydrodynamic flow conditions. Currently, we know little about the molecular details of this shear-stress-dependent interaction. Using single-molecule atomic force microscopy, we demonstrate that vWF binds to the S. aureus surface protein A (SpA) via a previously undescribed force-sensitive mechanism. We identify an extremely strong SpA-vWF interaction, capable of withstanding forces of ∼2 nN, both in laboratory and in clinically relevant methicillin-resistant S. aureus (MRSA) strains. Strong bonds are activated by mechanical stress, consistent with flow experiments revealing that bacteria adhere in larger amounts to vWF surfaces when the shear rate is increased. We suggest that force-enhanced adhesion may involve conformational changes in vWF. Under force, elongation of vWF may lead to the exposure of a high-affinity cryptic SpA-binding site to which bacteria firmly attach. In addition, force-induced structural changes in the SpA domains may also promote strong, high-affinity binding. This force-regulated interaction might be of medical importance as it may play a role in bacterial adherence to platelets and to damaged blood vessels.IMPORTANCEStaphylococcus aureus protein A (SpA) binds to von Willebrand factor (vWF) under flow. While vWF binding to SpA plays a role in S. aureus adherence to platelets and endothelial cells under shear stress, the molecular basis of this stress-dependent interaction has not yet been elucidated. Here we show that the SpA-vWF interaction is regulated by a new force-dependent mechanism. The results suggest that mechanical extension of vWF may lead to the exposure of a high-affinity cryptic SpA-binding site, consistent with the shear force-controlled functions of vWF. Moreover, strong binding may be promoted by force-induced structural changes in the SpA domains. This study highlights the role of mechanoregulation in controlling the adhesion of S. aureus and shows promise for the design of small inhibitors capable of blocking colonization under high shear stress.
Keywords: Staphylococcus aureus; adhesion; atomic force microscopy; mechanical force; von Willebrand factor.
Copyright © 2019 Viela et al.
Figures


Comment in
-
Bacterial pathogens under high-tension: Staphylococcus aureus adhesion to von Willebrand factor is activated by force.Microb Cell. 2019 Jun 11;6(7):321-323. doi: 10.15698/mic2019.07.684. Microb Cell. 2019. PMID: 31294044 Free PMC article.
References
-
- Fowler VG Jr, Miro JM, Hoen B, Cabell CH, Abrutyn E, Rubinstein E, Corey GR, Spelman D, Bradley SF, Barsic B, Pappas PA, Anstrom KJ, Wray D, Fortes CQ, Anguera I, Athan E, Jones P, van der Meer JTM, Elliott TSJ, Levine DP, Bayer AS, ICE Investigators. 2005. Staphylococcus aureus endocarditis: a consequence of medical progress. JAMA 293:3012–3021. doi:10.1001/jama.293.24.3012. - DOI - PubMed
-
- Pappelbaum KI, Gorzelanny C, Grässle S, Suckau J, Laschke MW, Bischoff M, Bauer C, Schorpp-Kistner M, Weidenmaier C, Schneppenheim R, Obser T, Sinha B, Schneider SW. 2013. Ultra-large von Willebrand factor fibers mediate luminal Staphylococcus aureus adhesion to an intact endothelial cell layer under shear stress. Circulation 128:50–59. doi:10.1161/CIRCULATIONAHA.113.002008. - DOI - PubMed
-
- Claes J, Vanassche T, Peetermans M, Liesenborghs L, Vandenbriele C, Vanhoorelbeke K, Missiakas D, Schneewind O, Hoylaerts MF, Heying R, Verhamme P. 2014. Adhesion of Staphylococcus aureus to the vessel wall under flow is mediated by von Willebrand factor-binding protein. Blood 124:1669–1676. doi:10.1182/blood-2014-02-558890. - DOI - PMC - PubMed
-
- Claes J, Liesenborghs L, Peetermans M, Veloso TR, Missiakas D, Schneewind O, Mancini S, Entenza JM, Hoylaerts MF, Heying R, Verhamme P, Vanassche T. 2017. Clumping factor A, von Willebrand factor-binding protein and von Willebrand factor anchor Staphylococcus aureus to the vessel wall. J Thromb Haemost 15:1009–1019. doi:10.1111/jth.13653. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous
