Similar Neural Pathways Control Foraging in Mosquitoes and Worms
- PMID: 31040241
- PMCID: PMC6495376
- DOI: 10.1128/mBio.00656-19
Similar Neural Pathways Control Foraging in Mosquitoes and Worms
Abstract
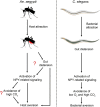
Female Aedes aegypti mosquitoes bite human hosts to obtain a blood meal and, in the process, act as vectors for many disease-causing viruses, including the dengue, chikungunya, yellow fever, and Zika viruses. After a complete meal, the female mosquitoes lose attraction to their hosts for several days. New research shows that pharmacological activation of neuropeptide Y-like receptor (NPYLR) signaling elicits host aversion in female mosquitoes. This behavior of mosquitoes shows remarkable similarities to a bacterial-aversion behavior of the nematode Caenorhabditis elegans Feeding on pathogenic bacteria causes bloating of the gut in C. elegans that leads to activation of NPYLR signaling and bacterial aversion. Several studies suggest that this newly discovered mechanism underlying foraging may be conserved across a large number of species. A better understanding of the regulation of NPYLR signaling pathways could provide molecular targets for the control of eating behaviors in different animals, including human-disease vectors.
Keywords: A. aegypti; C. elegans; NPR-1; aversion behavior; bacterial colonization; feeding; neuropeptide Y.
Copyright © 2019 Singh and Aballay.
Figures
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

