Buprenorphine/samidorphan combination for the adjunctive treatment of major depressive disorder: results of a phase III clinical trial (FORWARD-3)
- PMID: 31040679
- PMCID: PMC6459143
- DOI: 10.2147/NDT.S199245
Buprenorphine/samidorphan combination for the adjunctive treatment of major depressive disorder: results of a phase III clinical trial (FORWARD-3)
Abstract
Background: The endogenous opioid system is a fundamental regulator of mood in humans. Previously reported clinical trials have demonstrated the efficacy of the investigational agent buprenorphine/samidorphan (BUP/SAM) combination, an opioid-system modulator, for the adjunctive treatment of major depressive disorder. We present here a third phase III study of different design.
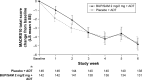
Methods: Adult patients with major depressive disorder and inadequate response to antidepressant therapy were enrolled in this double-blind, placebo-controlled, placebo run-in study to evaluate the efficacy, safety, and tolerability of adjunctive BUP/SAM 2 mg/2 mg. Patients with baseline Hamilton Depression Rating Scale score $20 received double-blind placebo in addition to background antidepressant therapy for 4 weeks. Nonresponders were randomized to receive adjunctive BUP/SAM 2 mg/2 mg or placebo for 6 weeks. The primary end point was change in Montgomery-Åsberg Depression Rating Scale (MADRS)-10 total score from randomization at baseline to the end of the 6-week treatment period.
Results: Least-squares mean change in MADRS-10 score at end of treatment was -4.8 (SE 0.67) in the BUP/SAM 2 mg/2 mg group and -4.6 (SE 0.66) in the placebo group (mean difference -0.3 [SE 0.95], P=0.782). There were no differences in MADRS-based response or remission rates. Overall, 42.9% of the BUP/SAM 2 mg/2 mg group and 34.5% of the placebo group experienced at least one treatment-emergent adverse event during the 6-week treatment period, most of which were mild or moderate in severity. There were no clinically important changes in laboratory parameters, weight, or vital signs and no evidence of abuse potential during treatment or opiate-withdrawal symptoms post treatment.
Conclusion: Efficacy results in FORWARD-3 measured by change in MADRS-10 score did not meet the primary end point, but postbaseline improvement in MADRS-10 in the BUP/SAM 2 mg/2 mg group was consistent with that seen in previously reported trials. BUP/SAM 2 mg/2 mg was well tolerated.
Keywords: adjunctive therapy; buprenorphine; opioid system modulator; placebo response; randomized clinical trial; samidorphan; study design.
Conflict of interest statement
Disclosure JMZ has received grants/research support from Actavis, Alkermes, Inc., Allergan, Axsome, elminda, Forest, the Cheryl T Herman Foundation, Hoffman-La Roche, Intra-Cellular Therapies, Janssen, Johnson & Johnson, Lundbeck, Otsuka, National Institutes of Health, Taisho, and Takeda, and has served as a consultant/advisory board participant for Alkermes, Inc., elminda, Lundbeck, and Takeda. ADS, AM, WFM, and SP are employees of Alkermes, Inc. The authors report no other conflicts of interest in this work.
Figures


References
-
- Knoth RL, Bolge SC, Kim E, Tran QV. Effect of inadequate response to treatment in patients with depression. Am J Manag Care. 2010;16(8):e188–e196. - PubMed
-
- Otte C, Gold SM, Penninx BW, et al. Major depressive disorder. Nat Rev Dis Primers. 2016;2:16065. - PubMed
-
- Bauer M, Bschor T, Pfennig A, et al. World Federation of Societies of Biological Psychiatry (WFSBP) guidelines for biological treatment of unipolar depressive disorders in primary care. World J Biol Psychiatry. 2007;8(2):67–104. - PubMed
LinkOut - more resources
Full Text Sources

